Gastrointestinal Endoscopy ( IF 6.7 ) Pub Date : 2022-09-07 , DOI: 10.1016/j.gie.2022.08.041
Se Woo Park 1 , Kyong Joo Lee 1 , Moon Jae Chung 2 , Jung Hyun Jo 2 , Hee Seung Lee 2 , Jeong Youp Park 2 , Seung Woo Park 2 , Si Young Song 2 , Huapyong Kang 3 , Eui Joo Kim 3 , Yeon Suk Kim 3 , Jae Hee Cho 4 , Seungmin Bang 2
|
Background and Aims
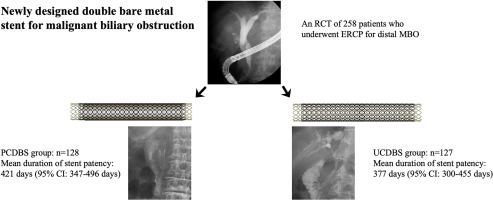
In a recent randomized controlled trial, a double bare metal stent (DBS) showed better stent patency than single-layer metal stents. However, clear evidence comparing the efficacy of uncovered (UCDBS) and partially covered (PCDBS) DBSs for distal malignant biliary obstruction (MBO) is lacking. Therefore, we compared the clinical outcomes including stent patency of UCDBSs versus PCDBSs.
Methods
A multicenter, randomized study was performed in patients with distal MBO. The primary endpoint was stent patency. Secondary endpoints were the proportion of patients with patent stents at 6 months, risk factors for stent dysfunction, overall survival, technical and clinical success rates of stent placement, and other adverse events (AEs).
Results
Among 258 included patients, 130 were randomly assigned to the PCDBS group and 128 to the UCDBS group. The mean duration of stent patency of the PCDBS (421.2 days; 95% confidence interval [CI], 346.7-495.7) was longer than that of the UCDBS (377.4 days; 95% CI, 299.7-455.0), although total stent dysfunction and stent dysfunction within 6 months were not different between groups. Multivariate analysis indicated that chemotherapy after stent placement was a significant factor for overall survival (hazard ratio, .570; 95% CI, .408-.796) and had a marginal impact on stent patency (hazard ratio, 1.569; 95% CI, .923-2.667). There were no remarkable differences in AEs, including pancreatitis, cholecystitis, and stent migration, between the 2 groups.
Conclusions
The use of PCDBSs compared with UCDBSs in patients with distal MBO has unclear benefits regarding stent patency and overall survival, although PCDBSs have a lower rate of tumor ingrowth. (Clinical trial registration number: NCT 02937246.)
中文翻译:

用于缓解不可切除的肝外恶性胆道梗阻的覆盖与未覆盖双裸自膨胀金属支架:一项随机对照多中心试验
背景和目标
在最近的一项随机对照试验中,双层裸金属支架 (DBS) 显示出比单层金属支架更好的支架通畅性。然而,比较未覆盖 (UCDBS) 和部分覆盖 (PCDBS) DBS 治疗远端恶性胆道梗阻 (MBO) 的疗效的明确证据尚不存在。因此,我们比较了 UCDBS 与 PCDBS 的临床结果,包括支架通畅率。
方法
在远端 MBO 患者中进行了一项多中心、随机研究。主要终点是支架通畅率。次要终点是 6 个月时有专利支架的患者比例、支架功能障碍的危险因素、总生存期、支架置入的技术和临床成功率以及其他不良事件 (AE)。
结果
在纳入的 258 名患者中,130 名被随机分配到 PCDBS 组,128 名被随机分配到 UCDBS 组。PCDBS 的平均支架通畅持续时间(421.2 天;95% 置信区间 [CI],346.7-495.7)长于 UCDBS(377.4 天;95% CI,299.7-455.0),尽管总体支架功能障碍和6 个月内支架功能障碍在组间没有差异。多变量分析表明,支架置入后化疗是影响总生存率的一个重要因素(风险比,0.570;95% CI,0.408-0.796),对支架通畅率的影响很小(风险比,1.569;95% CI, .923-2.667)。两组之间的不良事件(包括胰腺炎、胆囊炎和支架移位)没有显着差异。
结论
与 UCDBS 相比,在远端 MBO 患者中使用 PCDBS 对支架通畅率和总生存率的益处尚不明确,尽管 PCDBS 的肿瘤向内生长率较低。(临床试验注册号:NCT 02937246。)






































 京公网安备 11010802027423号
京公网安备 11010802027423号