当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sustainable Synthesis of Dual Single-Atom Catalyst of Pd?N4/Cu?N4 for Partial Oxidation of Ethylene Glycol
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2022-09-09 , DOI: 10.1002/adfm.202206887
Endalkachew Asefa Moges, Chia-Yu Chang, Wei-Hsiang Huang, Keseven Lakshmanan, Yohannes Ayele Awoke, Chih-Wen Pao, Meng-Che Tsai, Wei-Nien Su, Bing Joe Hwang
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2022-09-09 , DOI: 10.1002/adfm.202206887
Endalkachew Asefa Moges, Chia-Yu Chang, Wei-Hsiang Huang, Keseven Lakshmanan, Yohannes Ayele Awoke, Chih-Wen Pao, Meng-Che Tsai, Wei-Nien Su, Bing Joe Hwang
|
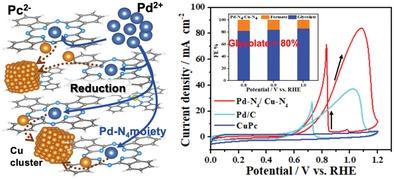
Catalysts assumed that properly designed bimetallic systems would provide superior catalytic performance due to the cooperative effects between two atoms. Dual single-atom catalyst (DSAC) PdN4/CuN4 is synthesized using a simple, cost-effective, and efficient electrochemical reduction method. The palladium single-atom is prepared first by electrochemical reduction of copper phthalocyanine to create defective N4 sites. The new structural feature is characterized by copper reduction from Cu-N4 coordination and the formation of defected N4 (▫M-N4) sites, which react with a Pd precursor and form PdN4 on the host surface. The DSAC PdN4/CuN4 technique synergistically improves electrocatalytic performance toward the ethylene glycol oxidation reaction. It possesses excellent glycolate selectivity (above 88%) in an alkaline solution with an onset oxidation potential as low as 0.6 V versus a reversible hydrogen electrode, compared to commercial Pd/C. The DSAC electrocatalyst is characterized by its high current density of 83.92 mA cm−2 and high faradic efficiency value (>80%) for glycolate at 1.0 VRHE. The findings suggest a promising method to synthesize the DSACs in varying transition metals to achieve highly efficient, selective, and environmentally friendly catalysts for different applications.
中文翻译:

用于乙二醇部分氧化的 Pd?N4/Cu?N4 双单原子催化剂的可持续合成
催化剂认为,由于两个原子之间的协同作用,适当设计的双金属系统将提供卓越的催化性能。双单原子催化剂 (DSAC) Pd N 4 /Cu N 4采用简单、经济高效的电化学还原方法合成。首先通过电化学还原铜酞菁以产生有缺陷的 N 4位点来制备钯单原子。新的结构特征的特点是铜从 Cu-N 4配位还原并形成有缺陷的 N 4 (▫ M -N 4 ) 位点,该位点与 Pd 前体反应并形成 Pd N 4在主体表面上。DSAC Pd N 4 /Cu N 4技术协同提高了乙二醇氧化反应的电催化性能。与商业 Pd/C 相比,它在碱性溶液中具有优异的乙醇酸选择性(高于 88%),与可逆氢电极相比,起始氧化电位低至 0.6 V。DSAC 电催化剂的特点是其 83.92 mA cm -2的高电流密度和在 1.0 V RHE下对乙醇酸盐的高法拉第效率值 (>80%). 研究结果提出了一种很有前景的方法,可以在不同的过渡金属中合成 DSAC,从而获得适用于不同应用的高效、选择性和环境友好型催化剂。
更新日期:2022-09-09
中文翻译:

用于乙二醇部分氧化的 Pd?N4/Cu?N4 双单原子催化剂的可持续合成
催化剂认为,由于两个原子之间的协同作用,适当设计的双金属系统将提供卓越的催化性能。双单原子催化剂 (DSAC) Pd N 4 /Cu N 4采用简单、经济高效的电化学还原方法合成。首先通过电化学还原铜酞菁以产生有缺陷的 N 4位点来制备钯单原子。新的结构特征的特点是铜从 Cu-N 4配位还原并形成有缺陷的 N 4 (▫ M -N 4 ) 位点,该位点与 Pd 前体反应并形成 Pd N 4在主体表面上。DSAC Pd N 4 /Cu N 4技术协同提高了乙二醇氧化反应的电催化性能。与商业 Pd/C 相比,它在碱性溶液中具有优异的乙醇酸选择性(高于 88%),与可逆氢电极相比,起始氧化电位低至 0.6 V。DSAC 电催化剂的特点是其 83.92 mA cm -2的高电流密度和在 1.0 V RHE下对乙醇酸盐的高法拉第效率值 (>80%). 研究结果提出了一种很有前景的方法,可以在不同的过渡金属中合成 DSAC,从而获得适用于不同应用的高效、选择性和环境友好型催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号