当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
General and Practical Route to Diverse 1-(Difluoro)alkyl-3-aryl Bicyclo[1.1.1]pentanes Enabled by an Fe-Catalyzed Multicomponent Radical Cross-Coupling Reaction
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-08 , DOI: 10.1021/acscatal.2c03498 Angel Rentería-Gómez 1 , Wes Lee 2 , Shuai Yin 1 , Michael Davis 2 , Achyut Ranjan Gogoi 1 , Osvaldo Gutierrez 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-08 , DOI: 10.1021/acscatal.2c03498 Angel Rentería-Gómez 1 , Wes Lee 2 , Shuai Yin 1 , Michael Davis 2 , Achyut Ranjan Gogoi 1 , Osvaldo Gutierrez 1, 2
Affiliation
|
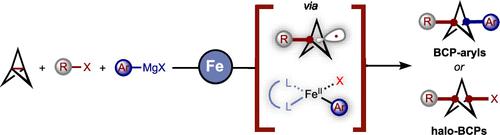
Bicyclo[1.1.1]pentanes (BCPs) are of great interest to the agrochemical, materials, and pharmaceutical industries. In particular, synthetic methods to access 1,3-dicarbosubsituted BCP-aryls have recently been developed, but most protocols rely on the stepwise C–C bond formation via the initial manipulation of BCP core to make the BCP electrophile or nucleophile followed by a second step (e.g., transition-metal-mediated cross-coupling step) to form the second key BCP-aryl bond. Moreover, despite the prevalence of C–F bonds in bioactive compounds, one-pot, multicomponent cross-coupling methods to directly functionalize [1.1.1]propellane to the corresponding fluoroalkyl BCP-aryl scaffolds are lacking. In this work, we describe a conceptually different approach to access diverse (fluoro)alkyl BCP-aryls at low temperatures and fast reaction times enabled by an iron-catalyzed multicomponent radical cross-coupling reaction from readily available (fluoro)alkyl halides, [1.1.1]propellane, and Grignard reagents. Further, experimental and computational mechanistic studies provide insights into the mechanism and ligand effects on the nature of C–C bond formation. Finally, these studies are used to develop a method to rapidly access synthetic versatile (difluoro)alkyl BCP halides via bisphosphine-iron catalysis.
中文翻译:

通过 Fe 催化的多组分自由基交叉偶联反应实现多样化的 1-(二氟)烷基-3-芳基双环[1.1.1]戊烷的一般和实用路线
双环[1.1.1]戊烷 (BCP) 对农用化学品、材料和制药行业非常感兴趣。特别是,最近开发了获得 1,3-二碳取代的 BCP-芳基的合成方法,但大多数方案依赖于通过 BCP 核心的初始操作逐步形成 C-C 键,以使 BCP 亲电试剂或亲核试剂,然后进行第二步(例如,过渡金属介导的交叉偶联步骤)以形成第二个关键 BCP-芳基键。此外,尽管 C-F 键在生物活性化合物中普遍存在,但缺乏将 [1.1.1] 推进烷直接官能化为相应的氟烷基 BCP-芳基支架的一锅法、多组分交叉偶联方法。在这项工作中,我们描述了一种概念上不同的方法,该方法通过来自现成的(氟)烷基卤化物、[1.1.1]推进烷和格氏试剂的铁催化多组分自由基交叉偶联反应,在低温和快速反应时间内获得不同的(氟)烷基 BCP-芳基。此外,实验和计算机理研究提供了对 C-C 键形成性质的机制和配体效应的见解。最后,这些研究用于开发一种通过双膦铁催化快速获得合成多功能(二氟)烷基 BCP 卤化物的方法。
更新日期:2022-09-08
中文翻译:

通过 Fe 催化的多组分自由基交叉偶联反应实现多样化的 1-(二氟)烷基-3-芳基双环[1.1.1]戊烷的一般和实用路线
双环[1.1.1]戊烷 (BCP) 对农用化学品、材料和制药行业非常感兴趣。特别是,最近开发了获得 1,3-二碳取代的 BCP-芳基的合成方法,但大多数方案依赖于通过 BCP 核心的初始操作逐步形成 C-C 键,以使 BCP 亲电试剂或亲核试剂,然后进行第二步(例如,过渡金属介导的交叉偶联步骤)以形成第二个关键 BCP-芳基键。此外,尽管 C-F 键在生物活性化合物中普遍存在,但缺乏将 [1.1.1] 推进烷直接官能化为相应的氟烷基 BCP-芳基支架的一锅法、多组分交叉偶联方法。在这项工作中,我们描述了一种概念上不同的方法,该方法通过来自现成的(氟)烷基卤化物、[1.1.1]推进烷和格氏试剂的铁催化多组分自由基交叉偶联反应,在低温和快速反应时间内获得不同的(氟)烷基 BCP-芳基。此外,实验和计算机理研究提供了对 C-C 键形成性质的机制和配体效应的见解。最后,这些研究用于开发一种通过双膦铁催化快速获得合成多功能(二氟)烷基 BCP 卤化物的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号