Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2022-09-08 , DOI: 10.1016/j.bmcl.2022.128978 Man-Yu Lin 1 , Tang-Yang Ji 1 , Miao Zheng 1 , Yan-Yan Chen 1 , Shi-Yi Xu 1 , Wen-Wei You 1 , Pei-Liang Zhao 1
|
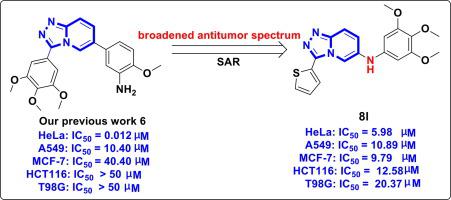
Based on our previous work, a series of novel 6-arylamino-[1,2,4]triazolo[4,3-a]pyridine derivatives were synthesized, and evaluated for antiproliferative activities. SAR studies revealed that inserting an amino linkage between 6‑aryl group and [1,2,4]triazolo[4,3-a]pyridine core led to a much broader antitumor spectrum, and the most promising compound 8 l exerted potent and broad-spectrum antiproliferative activity toward HeLa, HCT116, MCF-7, and A549 cell lines, with IC50 values in the micromolar range of 5.98–12.58 μM, which were more active than the positive control 5-FU. The mechanism investigation illustrated that 8 l dose-dependently caused cell cycle arrest at the G2/M phase, and induced cell apoptosis in HeLa cells. Consequently, these findings suggest the 6-arylamino-[1,2,4]triazolo[4,3-a]pyridines afford significant potential for the discovery of a new highly efficient anticancer agents.
中文翻译:

新型 6-芳氨基-[1,2,4]三唑并[4,3-a]吡啶衍生物作为抗增殖剂的高效合成与评价
基于我们之前的工作,合成了一系列新型 6-芳氨基-[1,2,4]三唑并[4,3- a ]吡啶衍生物,并对其抗增殖活性进行了评估。SAR研究表明,在6-芳基和[1,2,4]三唑并[4,3- a ]吡啶核心之间插入一个氨基键会导致 更广泛的 抗肿瘤 谱,最有希望的化合物8l发挥有效和 广泛的作用。 -对 HeLa、HCT116、MCF-7 和 A549 细胞系的谱抗增殖活性,IC 50值在 5.98–12.58 μM 的微摩尔范围内,比阳性对照 5-FU 更活跃。机理研究表明,8 l剂量依赖性地导致细胞周期停滞在 G 2 /M 期,并在 HeLa 细胞中诱导细胞凋亡。因此,这些发现表明,6-芳氨基-[1,2,4]三唑并[4,3- a ]吡啶为发现新型高效抗癌剂提供了巨大的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号