Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2022-09-08 , DOI: 10.1016/j.molstruc.2022.134103 Ziyue Xie , Zhengcheng Liang , Yunhou Huang , Kaichuang Shi , Ning Zang , Mian Wang , Taoyuan Liang , Wanxing Wei
|
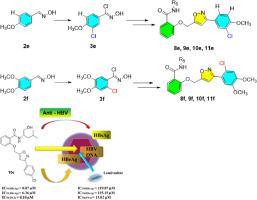
A series of 2-((3-phenylisoxazol-5-yl) methoxy) benzamides derivatives were designed and synthesized, and their anti-hepatitis B virus (HBV) activities were evaluated with HepG2.2.15 cells. The results showed that compounds 10a, 10c, 10d and 10f strongly inhibited secretion of HBeAg (IC50 = 0.20, 0.07, 0.30 and 0.12 μM, respectively, 3TC in 119.87 μM). Compounds 8a, 8b, 10c, 10f effectively inhibited HBV DNA replication with IC50 in 7.61, 8.92, 0.10 and 1.49 μM, respectively and were more effective than 3TC (IC50(DNA) = 13.02 μM). Result of docking studies indicated that compounds interacted to HBV core protein and coincided to their inhibitions on HBV. This work provided new compounds with significant inhibition on HBV for further optimization and development as potential non-nucleoside anti-virus agents. Meanwhile, chlorination on phenyl ring of dimethoxybenzaldehyde oxime occurred and did not follow Friedel - Crafts substitution when the oxime reacted with excess NCS.
中文翻译:

2-((3-phenylisoxazol-5-yl) methoxy) 苯甲酰胺衍生物作为强效核衣壳抑制剂的发现和生物学评价
设计合成了一系列2-((3-苯基异恶唑-5-基)甲氧基)苯甲酰胺衍生物,并用HepG2.2.15细胞评价其抗乙型肝炎病毒(HBV)活性。结果表明,化合物10a、10c、10d和10f强烈抑制 HBeAg 的分泌(IC 50 分别为 0.20、0.07、0.30 和 0.12 μM,3TC 在 119.87 μM)。化合物8a、8b、10c、10f有效抑制 HBV DNA 复制,IC 50分别为 7.61、8.92、0.10 和 1.49 μM,比 3TC (IC 50(DNA) = 13.02 微米)。对接研究结果表明,化合物与HBV核心蛋白相互作用,与它们对HBV的抑制作用相吻合。这项工作为进一步优化和开发作为潜在的非核苷类抗病毒剂提供了对HBV有显着抑制作用的新化合物。同时,二甲氧基苯甲醛肟与过量的NCS反应时,苯环发生氯化反应,且不遵循Friedel-Crafts取代反应。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号