当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-in-Class Hydrazide-Based HDAC6 Selective Inhibitor with Potent Oral Anti-Inflammatory Activity by Attenuating NLRP3 Inflammasome Activation
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-09-08 , DOI: 10.1021/acs.jmedchem.2c00853
Kairui Yue 1 , Simin Sun 1 , Geng Jia 1 , Mengting Qin 1 , Xiaohan Hou 1 , C James Chou 2 , Chao Huang 1 , Xiaoyang Li 1, 3
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-09-08 , DOI: 10.1021/acs.jmedchem.2c00853
Kairui Yue 1 , Simin Sun 1 , Geng Jia 1 , Mengting Qin 1 , Xiaohan Hou 1 , C James Chou 2 , Chao Huang 1 , Xiaoyang Li 1, 3
Affiliation
|
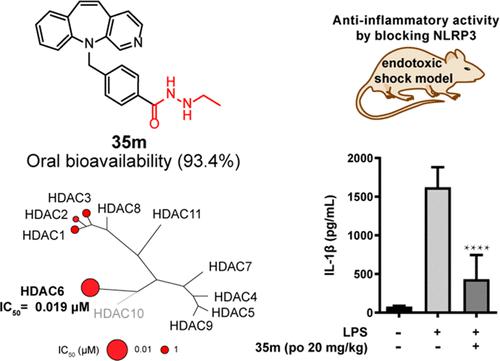
In this study, we report the first highly selective HDAC6 inhibitor with hydrazide as the zinc-binding group (ZBG), which displays superior pharmacokinetic properties to the current hydroxamic acid inhibitors. Structure–activity relationship study reveals that ethyl group substituent hydrazide-based ZBG and cap group with more substantial rigidity and larger volume increase the HDAC6 selectivity of designed compounds. Representative inhibitor 35m exhibits potent HDAC6 inhibitory activity with an IC50 value of 0.019 μM. To our surprise, 35m establishes significant improvement in the pharmacokinetic property with much higher AUC0-inf (10292 ng·h/mL) and oral bioavailability (93.4%) than hydroximic acid-based HDAC6 inhibitors Tubastatin A and ACY-1215. Low-dose 35m remarkably decreases LPS-induced IL-1β release both in vitro and in vivo by blocking the activation of NLRP3, indicating that 35m can be a potential orally active therapeutic agent for the treatment of NLRP3-related diseases.
中文翻译:

一流的基于酰肼的 HDAC6 选择性抑制剂,通过减弱 NLRP3 炎性体激活而具有有效的口服抗炎活性
在这项研究中,我们报告了第一个以酰肼为锌结合基团 (ZBG) 的高选择性 HDAC6 抑制剂,与目前的异羟肟酸抑制剂相比,它表现出优越的药代动力学特性。构效关系研究表明,乙基取代基酰肼基 ZBG 和具有更大刚性和更大体积的帽基增加了设计化合物的 HDAC6 选择性。代表性抑制剂35m表现出有效的 HDAC6 抑制活性,IC 50值为 0.019 μM。令我们惊讶的是,35m显着改善了药代动力学特性,AUC 0-inf更高(10292 ng·h/mL) 和口服生物利用度 (93.4%) 优于基于羟肟酸的 HDAC6 抑制剂 Tubastatin A 和 ACY-1215。低剂量35m通过阻断 NLRP3 的激活显着降低 LPS 诱导的 IL-1β在体外和体内的释放,表明35m可以成为治疗 NLRP3 相关疾病的潜在口服活性治疗剂。
更新日期:2022-09-08
中文翻译:

一流的基于酰肼的 HDAC6 选择性抑制剂,通过减弱 NLRP3 炎性体激活而具有有效的口服抗炎活性
在这项研究中,我们报告了第一个以酰肼为锌结合基团 (ZBG) 的高选择性 HDAC6 抑制剂,与目前的异羟肟酸抑制剂相比,它表现出优越的药代动力学特性。构效关系研究表明,乙基取代基酰肼基 ZBG 和具有更大刚性和更大体积的帽基增加了设计化合物的 HDAC6 选择性。代表性抑制剂35m表现出有效的 HDAC6 抑制活性,IC 50值为 0.019 μM。令我们惊讶的是,35m显着改善了药代动力学特性,AUC 0-inf更高(10292 ng·h/mL) 和口服生物利用度 (93.4%) 优于基于羟肟酸的 HDAC6 抑制剂 Tubastatin A 和 ACY-1215。低剂量35m通过阻断 NLRP3 的激活显着降低 LPS 诱导的 IL-1β在体外和体内的释放,表明35m可以成为治疗 NLRP3 相关疾病的潜在口服活性治疗剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号