Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-09-07 , DOI: 10.1016/j.apcatb.2022.121948
Honglin Li , Shoufu Cao , Hongman Sun , Yonglian Lu , Ying Zhang , Xiaoqing Lu , Jingbin Zeng , Zifeng Yan
|
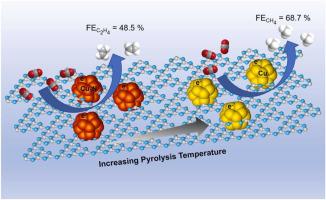
Copper (Cu) has been proved as an efficient catalyst in carbon dioxide electrochemical reduction reaction (CO2RR) towards hydrocarbons, but still suffers from low selectivity and poor stability. Herein, Cu-based/CxNy catalysts were fabricated by facile pyrolysis of CuNCN in sealed quartz tubes. It is found that CuNCN pyrolyzed at 300 °C (CuNCN-300) exhibits a high C2H4 Faradaic efficiency of 48.5% at 500 mA cm-2. However, increasing the pyrolysis temperature above 400 °C gives rise to CH4 being the predominant product and CuNCN-500 achieves CH4 Faradaic efficiencies of 66.3% at 300 mA cm-2. Combining experimental and DFT calculation results, Cu3N plays a crucial role in the formation of C2H4, while tri-s-triazine units in CuNCN-500 reduce the barrier of *CO hydrogenation to *CHO and retard C-C coupling on Cu surface. These findings mark the significance of precise tailoring of the synergistic effect between g-C3N4 and different Cu species for achieving the desired selectivity during CO2RR.
中文翻译:

CuNCN 衍生的 Cu 基/CxNy 催化剂用于高选择性 CO2 电还原成碳氢化合物
铜(Cu)已被证明是二氧化碳电化学还原反应(CO 2 RR)对碳氢化合物的有效催化剂,但仍存在选择性低、稳定性差的问题。在此,Cu 基/CxNy 催化剂是通过在密封石英管中简单地热解 CuNCN 来制造的。发现在 300 °C 热解的 CuNCN (CuNCN-300)在 500 mA cm - 2下表现出 48.5% 的高 C 2 H 4法拉第效率。然而,将热解温度提高到 400 °C 以上会导致 CH 4成为主要产物,并且 CuNCN-500在 300 mA cm - 2下实现了 66.3% 的 CH 4法拉第效率. 结合实验和DFT计算结果,Cu 3 N在C 2 H 4的形成中起着至关重要的作用,而CuNCN-500中的三-s-三嗪单元降低了*CO氢化成*CHO的势垒,并延缓了Cu上的CC偶联。表面。这些发现标志着精确调整gC 3 N 4和不同Cu 物种之间的协同效应对于在CO 2 RR期间实现所需选择性的重要性。



































 京公网安备 11010802027423号
京公网安备 11010802027423号