当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study on Nitrobenzene Hydrogenation by N-Doped Carbon-Supported Late Transition Metal Single-Atom Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-07 , DOI: 10.1021/acscatal.2c02373 Haohao Wang 1 , Fuxing Shi 1 , Min Pu 1 , Ming Lei 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-07 , DOI: 10.1021/acscatal.2c02373 Haohao Wang 1 , Fuxing Shi 1 , Min Pu 1 , Ming Lei 1
Affiliation
|
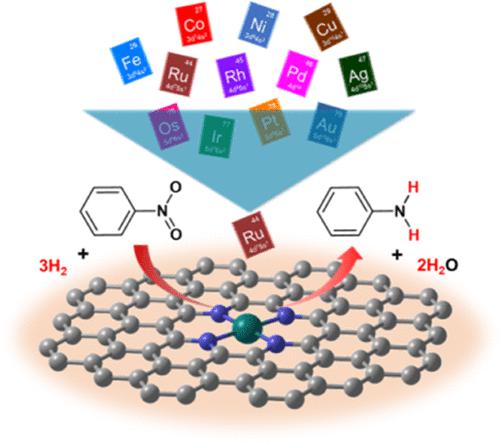
A density functional theory (DFT) study was performed to investigate the activities of 12 late transition metal single-atom-supported nitrogen-doped carbon-based catalysts (M1-N4/C SACs) nitrobenzene hydrogenation. Three different dihydrogen (H2) dissociation mechanisms are proposed: homolytic cleavage at the metal (M) site, heterolytic cleavage at M–N sites, and heterolytic cleavage at M–N–C sites. The coadsorption of nitrobenzene (PhNO2) and H2 was proposed, and the effect of charge change of hydrogen atoms caused by the coverage of PhNO2 on the H2 dissociation activity was revealed. Six M1–N4/C SACs (M = Ru, Os, Fe, Ag, Ir, Rh) with a potentially high activity of H2 dissociation were screened. Then, the two crucial steps, H2 dissociation and the second N–O bond breaking (PhNOH* + H* → PhN* + H2O), were compared. The obtained results indicate that different M1–N4/C SACs might have different rate-determining steps for nitrobenzene hydrogenation and that Ru1–N4/C SAC could be one of the most promising catalysts with good catalytic activities for nitrobenzene hydrogenation.
中文翻译:

N掺杂碳负载后过渡金属单原子催化剂对硝基苯加氢的理论研究
进行密度泛函理论 (DFT) 研究以研究 12 种后过渡金属单原子负载的氮掺杂碳基催化剂 (M 1 -N 4 /C SACs) 硝基苯加氢的活性。提出了三种不同的二氢 (H 2 ) 解离机制:金属 (M) 位点的均裂、M-N 位点的异裂和 M-N-C 位点的异裂。提出了硝基苯(PhNO 2 )与H 2的共吸附,揭示了PhNO 2的覆盖引起的氢原子电荷变化对H 2解离活性的影响。六个 M 1 –N 4筛选了具有潜在高 H 2解离活性的 /C SAC (M = Ru、Os、Fe、Ag、Ir、Rh)。然后,比较了两个关键步骤,H 2解离和第二个 N-O 键断裂(PhNOH* + H* → PhN* + H 2 O)。所得结果表明,不同的M 1 -N 4 /C SACs 可能对硝基苯加氢具有不同的限速步骤,Ru 1 -N 4 /C SAC 可能是最有前途的催化剂之一,具有良好的硝基苯加氢催化活性。
更新日期:2022-09-07
中文翻译:

N掺杂碳负载后过渡金属单原子催化剂对硝基苯加氢的理论研究
进行密度泛函理论 (DFT) 研究以研究 12 种后过渡金属单原子负载的氮掺杂碳基催化剂 (M 1 -N 4 /C SACs) 硝基苯加氢的活性。提出了三种不同的二氢 (H 2 ) 解离机制:金属 (M) 位点的均裂、M-N 位点的异裂和 M-N-C 位点的异裂。提出了硝基苯(PhNO 2 )与H 2的共吸附,揭示了PhNO 2的覆盖引起的氢原子电荷变化对H 2解离活性的影响。六个 M 1 –N 4筛选了具有潜在高 H 2解离活性的 /C SAC (M = Ru、Os、Fe、Ag、Ir、Rh)。然后,比较了两个关键步骤,H 2解离和第二个 N-O 键断裂(PhNOH* + H* → PhN* + H 2 O)。所得结果表明,不同的M 1 -N 4 /C SACs 可能对硝基苯加氢具有不同的限速步骤,Ru 1 -N 4 /C SAC 可能是最有前途的催化剂之一,具有良好的硝基苯加氢催化活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号