Cell Metabolism ( IF 27.7 ) Pub Date : 2022-09-06 , DOI: 10.1016/j.cmet.2022.08.007
Zhen Lu 1 , Noreen McBrearty 1 , Jinyun Chen 1 , Vivek S Tomar 1 , Hongru Zhang 1 , Gianluca De Rosa 1 , Aiwen Tan 2 , Aalim M Weljie 2 , Daniel P Beiting 3 , Zhen Miao 4 , Subin S George 5 , Allison Berger 6 , Gurpanna Saggu 6 , J Alan Diehl 7 , Constantinos Koumenis 8 , Serge Y Fuchs 1
|
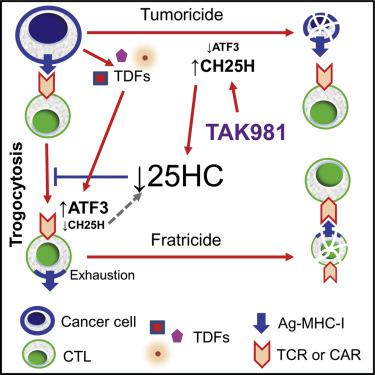
Effector trogocytosis between malignant cells and tumor-specific cytotoxic T lymphocytes (CTLs) contributes to immune evasion through antigen loss on target cells and fratricide of antigen-experienced CTLs by other CTLs. The mechanisms regulating these events in tumors remain poorly understood. Here, we demonstrate that tumor-derived factors (TDFs) stimulated effector trogocytosis and restricted CTLs’ tumoricidal activity and viability in vitro. TDFs robustly altered the CTL’s lipid profile, including depletion of 25-hydroxycholesterol (25HC). 25HC inhibited trogocytosis and prevented CTL’s inactivation and fratricide. Mechanistically, TDFs induced ATF3 transcription factor that suppressed the expression of 25HC-regulating gene—cholesterol 25-hydroxylase (CH25H). Stimulation of trogocytosis in the intratumoral CTL by the ATF3-CH25H axis attenuated anti-tumor immunity, stimulated tumor growth, and impeded the efficacy of chimeric antigen receptor (CAR) T cell adoptive therapy. Through use of armored CAR constructs or pharmacologic agents restoring CH25H expression, we reversed these phenotypes and increased the efficacy of immunotherapies.
中文翻译:

ATF3 和 CH25H 调节内源性和免疫治疗性细胞毒性 T 淋巴细胞的效应细胞作用和抗肿瘤活性
恶性细胞和肿瘤特异性细胞毒性 T 淋巴细胞 (CTL) 之间的效应器吞噬作用通过靶细胞上的抗原丢失以及其他 CTL 对经历过抗原的 CTL 的自相残杀而有助于免疫逃避。肿瘤中调节这些事件的机制仍然知之甚少。在这里,我们证明肿瘤衍生因子 (TDF) 刺激效应细胞吞噬作用并限制 CTL 的体外杀肿瘤活性和活力。 TDF 强烈改变了 CTL 的脂质谱,包括 25-羟基胆固醇 (25HC) 的消耗。 25HC抑制胞饮作用并防止CTL失活和自相残杀。从机制上讲,TDF 诱导 ATF3 转录因子抑制 25HC 调节基因——胆固醇 25-羟化酶 ( CH25H ) 的表达。 ATF3-CH25H 轴刺激肿瘤内 CTL 的吞噬作用减弱了抗肿瘤免疫,刺激肿瘤生长,并阻碍嵌合抗原受体 (CAR) T 细胞过继治疗的功效。通过使用装甲 CAR 构建体或恢复 CH25H 表达的药物,我们逆转了这些表型并提高了免疫疗法的功效。

































 京公网安备 11010802027423号
京公网安备 11010802027423号