当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β-MnO2 nanoparticles as heterogenous catalysts for aerobic oxidative transformation of alcohols to carbonyl compounds, nitriles, and amides
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2022-09-06 , DOI: 10.1039/d2cy01476a
Keigo Kamata 1 , Nanami Kinoshita 1 , Maki Koutani 1 , Ryusei Aono 1 , Eri Hayashi 1 , Michikazu Hara 1
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2022-09-06 , DOI: 10.1039/d2cy01476a
Keigo Kamata 1 , Nanami Kinoshita 1 , Maki Koutani 1 , Ryusei Aono 1 , Eri Hayashi 1 , Michikazu Hara 1
Affiliation
|
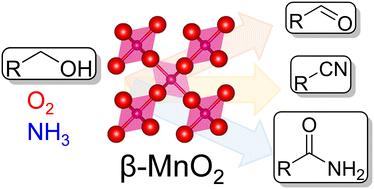
β-MnO2 nanoparticles with high specific surface areas were successfully synthesized by the crystallization of a layered manganese oxide precursor prepared using not only NaMnO4, but also inexpensive and easily available KMnO4. These β-MnO2 nanoparticles could function as an effective and reusable solid catalyst for the aerobic oxidation of various aromatic and heteroaromatic alcohols to the corresponding carbonyl compounds with molecular oxygen as the sole oxidant. β-MnO2 exhibited higher catalytic activity than other catalysts, including manganese-based simple and complex oxides under mild reaction conditions. In addition, the present oxidation system could be applied to the one-pot tandem oxidative transformation of alcohols to the corresponding nitriles and amides in the presence of ammonia, without the need for any additives such as strong bases and nitroxyl radicals. β-MnO2 nanoparticles were found to be more effective catalysts for the selective synthesis of nitriles from alcohols than the α-MnO2 based OMS-2 catalyst, likely due to the high oxidation activity and low nitrile hydration activity of β-MnO2. Mechanistic studies including the poisoning effects for nitrile hydration showed that the differences in acid sites and crystal structures between β-MnO2 and OMS-2 likely affect the activation of the nitriles and water, respectively, which leads to the high selectivity of β-MnO2 for nitrile synthesis.
中文翻译:

β-MnO2 纳米颗粒作为多相催化剂,用于醇类需氧氧化转化为羰基化合物、腈类和酰胺类
具有高比表面积的β-MnO 2纳米粒子通过结晶层状氧化锰前驱体成功合成,该前驱体不仅使用NaMnO 4制备,而且还使用廉价且易于获得的KMnO 4制备。这些 β-MnO 2纳米粒子可以作为一种有效且可重复使用的固体催化剂,用于以分子氧为唯一氧化剂将各种芳族和杂芳族醇有氧氧化为相应的羰基化合物。β-MnO 2在温和的反应条件下,表现出比其他催化剂更高的催化活性,包括锰基简单和复杂的氧化物。此外,本氧化体系可用于在氨存在下将醇一锅串联氧化转化为相应的腈和酰胺,而不需要任何添加剂,如强碱和硝酰基自由基。与基于α-MnO 2的OMS-2催化剂相比, β-MnO 2纳米颗粒被发现是从醇选择性合成腈的更有效催化剂,这可能是由于β-MnO 2的高氧化活性和低腈水合活性. 包括腈水合中毒效应在内的机理研究表明,β-MnO 2和 OMS-2 之间酸位和晶体结构的差异可能分别影响腈和水的活化,从而导致 β-MnO 的高选择性2用于腈合成。
更新日期:2022-09-06
中文翻译:

β-MnO2 纳米颗粒作为多相催化剂,用于醇类需氧氧化转化为羰基化合物、腈类和酰胺类
具有高比表面积的β-MnO 2纳米粒子通过结晶层状氧化锰前驱体成功合成,该前驱体不仅使用NaMnO 4制备,而且还使用廉价且易于获得的KMnO 4制备。这些 β-MnO 2纳米粒子可以作为一种有效且可重复使用的固体催化剂,用于以分子氧为唯一氧化剂将各种芳族和杂芳族醇有氧氧化为相应的羰基化合物。β-MnO 2在温和的反应条件下,表现出比其他催化剂更高的催化活性,包括锰基简单和复杂的氧化物。此外,本氧化体系可用于在氨存在下将醇一锅串联氧化转化为相应的腈和酰胺,而不需要任何添加剂,如强碱和硝酰基自由基。与基于α-MnO 2的OMS-2催化剂相比, β-MnO 2纳米颗粒被发现是从醇选择性合成腈的更有效催化剂,这可能是由于β-MnO 2的高氧化活性和低腈水合活性. 包括腈水合中毒效应在内的机理研究表明,β-MnO 2和 OMS-2 之间酸位和晶体结构的差异可能分别影响腈和水的活化,从而导致 β-MnO 的高选择性2用于腈合成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号