当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Resonance Stabilization Energy of 1,2-Azaborines: A Quantitative Experimental Study by Reaction Calorimetry
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-12-29 , DOI: 10.1021/ja109596m Patrick G. Campbell 1 , Eric R. Abbey 1 , Doinita Neiner 1 , Daniel J. Grant 1 , David A. Dixon 1 , Shih-Yuan Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-12-29 , DOI: 10.1021/ja109596m Patrick G. Campbell 1 , Eric R. Abbey 1 , Doinita Neiner 1 , Daniel J. Grant 1 , David A. Dixon 1 , Shih-Yuan Liu 1
Affiliation
|
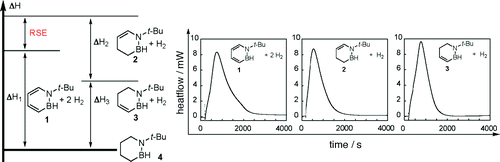
Aromatic and single-olefin six-membered BN heterocycles were synthesized, and the heats of hydrogenation were measured calorimetrically. A comparison of the hydrogenation enthalpies of these compounds revealed that 1,2-azaborines have a resonance stabilization energy of 16.6 ± 1.3 kcal/mol, in good agreement with calculated values.
中文翻译:

1,2-氮杂硼的共振稳定能:反应量热法的定量实验研究
合成了芳香族和单烯烃六元 BN 杂环,并通过量热法测量了氢化热。这些化合物的氢化焓的比较表明,1,2-氮杂硼烷的共振稳定能量为 16.6 ± 1.3 kcal/mol,与计算值非常吻合。
更新日期:2010-12-29
中文翻译:

1,2-氮杂硼的共振稳定能:反应量热法的定量实验研究
合成了芳香族和单烯烃六元 BN 杂环,并通过量热法测量了氢化热。这些化合物的氢化焓的比较表明,1,2-氮杂硼烷的共振稳定能量为 16.6 ± 1.3 kcal/mol,与计算值非常吻合。































 京公网安备 11010802027423号
京公网安备 11010802027423号