Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2022-09-05 , DOI: 10.1016/j.bmc.2022.116993 Rong Li 1 , Huilin Su 1 , Wei Chen 1 , Yu-Hang Yan 2 , Cong Zhou 2 , Luohe Mou 1 , Huan Yang 1 , Shan Qian 1 , Zhouyu Wang 3 , Lingling Yang 1 , Guo-Bo Li 2
|
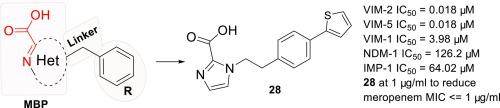
As one of important mechanisms to β-lactam antimicrobial resistance, metallo-β-lactamases (MBLs) have been receiving increasing worldwide attentions. Ambler subclass B1 MBLs are most clinically relevant, because they can hydrolyze almost all β-lactams with the exception of monobactams. However, it is still lacking of clinically useful drugs to combat MBL-medicated resistance. We previously identified 1H-imidazole-2-carboxylic acid as a core metal-binding pharmacophore (MBP) to target multiple B1 MBLs. Herein, we report structural optimization of 1H-imidazole-2-carboxylic acid and substituents. Structure-activity relationship (SAR) analyses revealed that replacement of 1H-imidazole-2-carboxylic acid with other structurally highly similar MBPs excepting thiazole-4-carboxylic acid resulted in decreased MBL inhibition. Further SAR studies identified more potent inhibitors to MBLs, of which 28 manifested IC50 values of 0.018 µM for both VIM-2 and VIM-5. The microbiological tests demonstrated that the most tested compounds showed improved synergistic effects; some compounds at 1 µg/ml were able to reduce meropenem MIC by at least 16-fold, which will be worth further development of new potent inhibitors particularly targeting VIM-type MBLs.
中文翻译:

作为金属-β-内酰胺酶抑制剂的新型 1H-咪唑-2-羧酸衍生物的设计、合成和生物学评价
作为β-内酰胺类抗菌素耐药的重要机制之一,金属-β-内酰胺酶(MBLs)越来越受到世界范围的关注。Ambler 亚类 B1 MBL 与临床最相关,因为它们可以水解几乎所有的 β-内酰胺,除了单环内酰胺。然而,它仍然缺乏临床上有用的药物来对抗 MBL 药物的耐药性。我们之前将 1 H-咪唑-2-羧酸确定为核心金属结合药效团 (MBP),以靶向多个 B1 MBL。在此,我们报告了 1 H-咪唑-2-羧酸和取代基的结构优化。构效关系 (SAR) 分析表明,取代 1 H- 咪唑-2-羧酸与除噻唑-4-羧酸外的其他结构高度相似的 MBP 导致 MBL 抑制降低。进一步的 SAR 研究确定了更有效的 MBL 抑制剂,其中28种对 VIM-2 和 VIM-5 的IC 50值均为 0.018 µM。微生物测试表明,测试最多的化合物显示出改善的协同作用;一些 1 µg/ml 的化合物能够将美罗培南的 MIC 降低至少 16 倍,这将值得进一步开发特别针对 VIM 型 MBL 的新型强效抑制剂。








































 京公网安备 11010802027423号
京公网安备 11010802027423号