当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cell-Membrane-Anchored DNA Logic-Gated Nanoassemblies for In Situ Extracellular Bioimaging
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-09-02 , DOI: 10.1021/acsami.2c13735
Hangsheng Gong 1 , Qian Dai 1 , Pai Peng 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-09-02 , DOI: 10.1021/acsami.2c13735
Hangsheng Gong 1 , Qian Dai 1 , Pai Peng 1
Affiliation
|
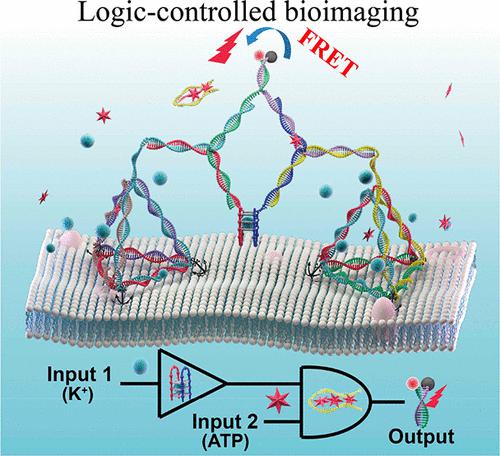
Extracellular K+ and adenosine triphosphate (ATP) levels are significantly elevated in the tumor microenvironment (TME) and can be used as biomarkers for early cancer detection and tumor localization. Most reported TME sensors only respond to single abnormal factors, resulting in a lack of accuracy and specificity for the detection of complex environments. Thus, precisely locating the TME remains challenging. In this work, we aimed to develop an intelligent DNA nanoassembly controlled by a “YES-AND” logic circuit using a bimolecular G-quadruplex (G4) and ATP aptamer as logical control units. As a proof of concept, in the presence of K+ (input 1) and ATP (input 2), the YES-AND Boolean operator returned a true value and the output was the fluorescence resonance energy transfer (FRET) signal, indicating high sensitivity and selectivity. After being anchored to living cell surfaces, this logic nanosensor imaged extracellular K+ and ATP present at abnormal levels in situ. Owing to diverse disease markers in the TME, this novel logic sensor might hold great promise for the targeted delivery of intelligent anticancer drugs and Boolean logic-controlled treatment.
中文翻译:

用于原位细胞外生物成像的细胞膜锚定 DNA 逻辑门控纳米组件
细胞外 K +和三磷酸腺苷 (ATP) 水平在肿瘤微环境 (TME) 中显着升高,可用作早期癌症检测和肿瘤定位的生物标志物。大多数报道的 TME 传感器仅对单一的异常因素做出响应,导致对复杂环境的检测缺乏准确性和特异性。因此,精确定位 TME 仍然具有挑战性。在这项工作中,我们旨在开发一种由“YES-AND”逻辑电路控制的智能 DNA 纳米组装体,使用双分子 G-四链体 (G4) 和 ATP 适体作为逻辑控制单元。作为概念证明,在存在 K +(输入 1)和 ATP(输入 2),YES-AND 布尔运算符返回真值,输出为荧光共振能量转移 (FRET) 信号,表明灵敏度和选择性很高。在锚定到活细胞表面后,这种逻辑纳米传感器可对细胞外 K +和 ATP 原位异常水平进行成像。由于 TME 中的多种疾病标志物,这种新型逻辑传感器可能为智能抗癌药物的靶向递送和布尔逻辑控制治疗带来巨大希望。
更新日期:2022-09-02
中文翻译:

用于原位细胞外生物成像的细胞膜锚定 DNA 逻辑门控纳米组件
细胞外 K +和三磷酸腺苷 (ATP) 水平在肿瘤微环境 (TME) 中显着升高,可用作早期癌症检测和肿瘤定位的生物标志物。大多数报道的 TME 传感器仅对单一的异常因素做出响应,导致对复杂环境的检测缺乏准确性和特异性。因此,精确定位 TME 仍然具有挑战性。在这项工作中,我们旨在开发一种由“YES-AND”逻辑电路控制的智能 DNA 纳米组装体,使用双分子 G-四链体 (G4) 和 ATP 适体作为逻辑控制单元。作为概念证明,在存在 K +(输入 1)和 ATP(输入 2),YES-AND 布尔运算符返回真值,输出为荧光共振能量转移 (FRET) 信号,表明灵敏度和选择性很高。在锚定到活细胞表面后,这种逻辑纳米传感器可对细胞外 K +和 ATP 原位异常水平进行成像。由于 TME 中的多种疾病标志物,这种新型逻辑传感器可能为智能抗癌药物的靶向递送和布尔逻辑控制治疗带来巨大希望。




































 京公网安备 11010802027423号
京公网安备 11010802027423号