当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Spiro[Azetidine-3,3′-Indoline]-2,2′-Diones via Copper(I)-Catalyzed Kinugasa/C−C Coupling Cascade Reaction
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-09-02 , DOI: 10.1002/anie.202208323 Xianqiang Zhong 1 , Meirong Huang 2 , Huilan Xiong 1 , Yuzhen Liang 1 , Wei Zhou 1 , Qian Cai 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-09-02 , DOI: 10.1002/anie.202208323 Xianqiang Zhong 1 , Meirong Huang 2 , Huilan Xiong 1 , Yuzhen Liang 1 , Wei Zhou 1 , Qian Cai 1
Affiliation
|
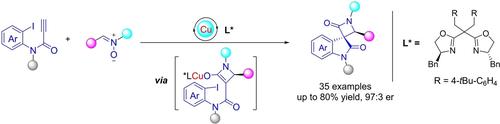
Spiro[azetidine-indolines] are important scaffolds in diversified bioactive compounds, but asymmetric synthesis of chiral spiro[azetidine-3,3′-indolines] remains unexplored. In this work, a copper-catalyzed asymmetric Kinugasa/C−C coupling cascade reaction of N-(2-iodo-aryl)-propiolamides with nitrones has been developed, affording chiral spiro[azetidine-3,3′-indoline]-2,2′-diones as single diastereomers with high yields and good enantioselectivity.
中文翻译:

螺[Azetidine-3,3'-Indoline]-2,2'-Diones 通过铜 (I)-催化的 Kinugasa/C−C 偶联级联反应的不对称合成
螺[azetidine-indolines] 是多种生物活性化合物的重要支架,但手性螺[azetidine-3,3'-indolines] 的不对称合成仍未探索。在这项工作中,开发了N -(2-iodo-aryl)-propiolamides 与硝酮的铜催化不对称 Kinugasa/CC 偶联级联反应,提供手性螺 [azetidine-3,3'-indoline]-2 ,2'-二酮作为单一非对映体,具有高产率和良好的对映选择性。
更新日期:2022-09-02
中文翻译:

螺[Azetidine-3,3'-Indoline]-2,2'-Diones 通过铜 (I)-催化的 Kinugasa/C−C 偶联级联反应的不对称合成
螺[azetidine-indolines] 是多种生物活性化合物的重要支架,但手性螺[azetidine-3,3'-indolines] 的不对称合成仍未探索。在这项工作中,开发了N -(2-iodo-aryl)-propiolamides 与硝酮的铜催化不对称 Kinugasa/CC 偶联级联反应,提供手性螺 [azetidine-3,3'-indoline]-2 ,2'-二酮作为单一非对映体,具有高产率和良好的对映选择性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号