当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable Process of Spiro(cyclopropane)oxazepane Pyridine Carboxylic Acid through Kulinkovich, Mitsunobu, and Pd-Catalyzed Intramolecular C–N Coupling
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-09-01 , DOI: 10.1021/acs.oprd.2c00221 Bo Qu 1 , Jaehee Lee 1 , Lifen Wu 1 , Anjan K. Saha 1 , Guisheng Li 1 , Yibo Xu 1 , Zhulin Tan 1 , Jason Brazzillo 1 , Nizar Haddad 1 , Scott Pennino 2 , Sonia Rodriguez 1 , Jon C. Lorenz 1 , Rogelio Frutos 1 , Kanwar P. S. Sidhu 1 , Xiao-Jun Wang 1 , Yongda Zhang 1 , Chris H. Senanayake 1 , Jinhua J. Song 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-09-01 , DOI: 10.1021/acs.oprd.2c00221 Bo Qu 1 , Jaehee Lee 1 , Lifen Wu 1 , Anjan K. Saha 1 , Guisheng Li 1 , Yibo Xu 1 , Zhulin Tan 1 , Jason Brazzillo 1 , Nizar Haddad 1 , Scott Pennino 2 , Sonia Rodriguez 1 , Jon C. Lorenz 1 , Rogelio Frutos 1 , Kanwar P. S. Sidhu 1 , Xiao-Jun Wang 1 , Yongda Zhang 1 , Chris H. Senanayake 1 , Jinhua J. Song 1
Affiliation
|
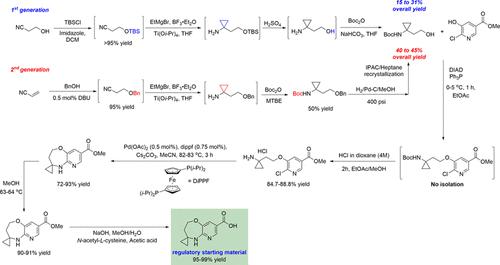
The oxazepane pyridine intermediate, an important fragment of active pharmaceutical ingredients, is of great interest in the pharmaceutical industry. In this manuscript, a scalable and economical process for the synthesis of a fused 2′,3′-dihydro-5’H-spiro[cyclopropane-1,4′-pyrido[3,2-b][1,4]oxazepine]-8′-carboxylic acid 1 on multikilogram scales is described. The synthesis features a streamlined isolation process from the Mitsunobu reaction by using one solvent system for a two-step process. Furthermore, a robust palladium-catalyzed intramolecular amination for the seven-membered heterocycle was developed. The reproducible reaction rate was established by adding the catalyst and ligand as solids without preparing the palladium complex under nitrogen. The residual palladium was effectively reduced by recrystallization of the carboxylic ester intermediate 2. The synthesis of key intermediate 5 was realized via the Kulinkovich reaction from readily available simple building blocks. The regulatory starting material compound 1 was isolated in a high-purity profile after saponification of the ester 2 with an overall yield of 70% over five steps.
中文翻译:

通过 Kulinkovich、Mitsunobu 和 Pd 催化的分子内 C-N 偶联制备螺(环丙烷)氧氮杂吡啶羧酸的可扩展过程
氧氮杂吡啶中间体是活性药物成分的重要片段,在制药工业中引起了极大的兴趣。在这篇手稿中,一种可扩展且经济的合成 2',3'-dihydro-5' H-螺[环丙烷-1,4'-吡啶并[3,2-b][1,4]恶氮平的方法]-8'-羧酸1描述了多公斤的规模。该合成的特点是通过使用一种溶剂系统进行两步工艺,从 Mitsunobu 反应中分离出流线型的工艺。此外,开发了一种用于七元杂环的强大的钯催化分子内胺化。通过添加固体形式的催化剂和配体而不在氮气下制备钯络合物来建立可再现的反应速率。通过羧酸酯中间体2的重结晶有效地减少了残余钯。关键中间体5的合成是通过 Kulinkovich 反应从容易获得的简单构件中实现的。监管起始材料化合物1在酯2皂化后以高纯度分离得到,经过五步总收率为 70%。
更新日期:2022-09-01
中文翻译:

通过 Kulinkovich、Mitsunobu 和 Pd 催化的分子内 C-N 偶联制备螺(环丙烷)氧氮杂吡啶羧酸的可扩展过程
氧氮杂吡啶中间体是活性药物成分的重要片段,在制药工业中引起了极大的兴趣。在这篇手稿中,一种可扩展且经济的合成 2',3'-dihydro-5' H-螺[环丙烷-1,4'-吡啶并[3,2-b][1,4]恶氮平的方法]-8'-羧酸1描述了多公斤的规模。该合成的特点是通过使用一种溶剂系统进行两步工艺,从 Mitsunobu 反应中分离出流线型的工艺。此外,开发了一种用于七元杂环的强大的钯催化分子内胺化。通过添加固体形式的催化剂和配体而不在氮气下制备钯络合物来建立可再现的反应速率。通过羧酸酯中间体2的重结晶有效地减少了残余钯。关键中间体5的合成是通过 Kulinkovich 反应从容易获得的简单构件中实现的。监管起始材料化合物1在酯2皂化后以高纯度分离得到,经过五步总收率为 70%。































 京公网安备 11010802027423号
京公网安备 11010802027423号