当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroamination of Unactivated Alkenes with Aliphatic Azides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-01 , DOI: 10.1021/jacs.2c07643 Si-Ming Jia 1 , Yi-Hang Huang 1 , Zhan-Lin Wang 1 , Fang-Xu Fan 1 , Bo-Han Fan 1 , Hao-Xiang Sun 1 , Hao Wang 1 , Fei Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-01 , DOI: 10.1021/jacs.2c07643 Si-Ming Jia 1 , Yi-Hang Huang 1 , Zhan-Lin Wang 1 , Fang-Xu Fan 1 , Bo-Han Fan 1 , Hao-Xiang Sun 1 , Hao Wang 1 , Fei Wang 1
Affiliation
|
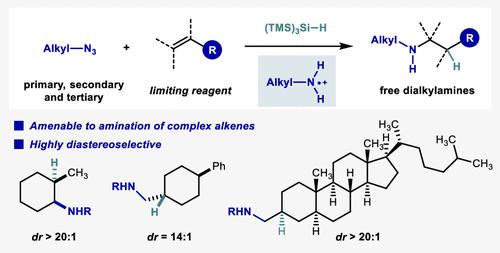
We report here an efficient and highly diastereoselective intermolecular anti-Markovnikov hydroamination of unactivated alkenes with aliphatic azides in the presence of silane. The system tolerates a wide range of azides and alkenes and operates with alkene as limiting reagent. Mechanistic studies suggest a radical chain pathway that involves aminium radical formation, radical addition to alkenes and HAT from silane to β-aminium alkyl radical. The use of sterically bulky silane is proposed to contribute to the excellent diastereoselectivity for HAT. Computational analysis uncovers the reaction pathway of aliphatic azide activation with silyl radical for aminyl radical formation.
中文翻译:

未活化烯烃与脂肪族叠氮化物的加氢胺化
我们在此报告了在硅烷存在下用脂肪族叠氮化物对未活化烯烃进行有效且高度非对映选择性的分子间抗马尔科夫尼科夫加氢胺化。该系统可耐受多种叠氮化物和烯烃,并使用烯烃作为限制试剂。机理研究表明,自由基链途径包括胺自由基的形成、烯烃的自由基加成和从硅烷到 β-胺烷基自由基的 HAT。建议使用空间大的硅烷有助于 HAT 具有出色的非对映选择性。计算分析揭示了脂肪族叠氮化物与甲硅烷基自由基活化形成胺基自由基的反应途径。
更新日期:2022-09-01
中文翻译:

未活化烯烃与脂肪族叠氮化物的加氢胺化
我们在此报告了在硅烷存在下用脂肪族叠氮化物对未活化烯烃进行有效且高度非对映选择性的分子间抗马尔科夫尼科夫加氢胺化。该系统可耐受多种叠氮化物和烯烃,并使用烯烃作为限制试剂。机理研究表明,自由基链途径包括胺自由基的形成、烯烃的自由基加成和从硅烷到 β-胺烷基自由基的 HAT。建议使用空间大的硅烷有助于 HAT 具有出色的非对映选择性。计算分析揭示了脂肪族叠氮化物与甲硅烷基自由基活化形成胺基自由基的反应途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号