当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Role of Chemical Reactions in the Selectivity of Tyrosine Kinase Inhibitors
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-31 , DOI: 10.1021/jacs.2c07307 Mojgan Asadi 1 , Wen Jun Xie 1 , Arieh Warshel 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-31 , DOI: 10.1021/jacs.2c07307 Mojgan Asadi 1 , Wen Jun Xie 1 , Arieh Warshel 1
Affiliation
|
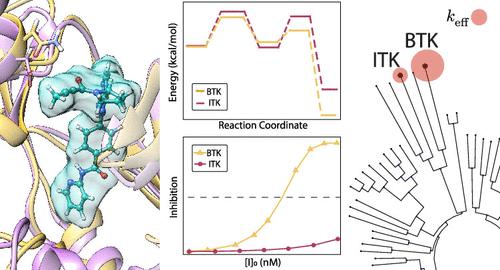
A variety of diseases are associated with tyrosine kinase enzymes that activate many proteins via signal transduction cascades. The similar ATP-binding pockets of these tyrosine kinases make it extremely difficult to design selective covalent inhibitors. The present study explores the contribution of the chemical reaction steps to the selectivity of the commercialized inhibitor acalabrutinib over the Bruton’s tyrosine kinase (BTK) and the interleukin-2-inducible T-cell kinase (ITK). Ab initio and empirical valence bond (EVB) simulations of the two kinases indicate that the most favorable reaction path involves a water-assisted mechanism of the 2-butynamide reactive group of acalabrutinib. BTK reacts with acalabrutinib with a substantially lower barrier than ITK, according to our calculated free-energy profile and kinetic simulations. Such a difference is due to the microenvironment of the active site, as further supported by a sequence-based analysis of specificity determinants for several commercialized inhibitors. Our study involves a new approach of simulating directly the IC50 and inactivation efficiency keff, instead of using the standard formulas. This new strategy is particularly important in studies of covalent inhibitors with a very exothermic bonding step. Overall, our results demonstrate the importance of understanding the chemical reaction steps in designing selective covalent inhibitors for tyrosine kinases.
中文翻译:

探索化学反应在酪氨酸激酶抑制剂选择性中的作用
许多疾病都与酪氨酸激酶有关,酪氨酸激酶通过信号转导级联激活许多蛋白质。这些酪氨酸激酶相似的 ATP 结合袋使得设计选择性共价抑制剂变得极其困难。本研究探讨了化学反应步骤对商业化抑制剂 acalabrutinib 相对于布鲁顿酪氨酸激酶 (BTK) 和白细胞介素 2 诱导型 T 细胞激酶 (ITK) 的选择性的贡献。两种激酶的从头计算和经验价键 (EVB) 模拟表明,最有利的反应路径涉及 acalabrutinib 的 2-丁酰胺反应基团的水辅助机制。根据我们计算的自由能曲线和动力学模拟,BTK 与 acalabrutinib 发生反应的势垒比 ITK 低得多。这种差异是由于活性位点的微环境造成的,对几种商业化抑制剂的特异性决定因素的基于序列的分析进一步支持了这一点。我们的研究涉及一种直接模拟 IC50 和灭活效率k eff的新方法,而不是使用标准公式。这种新策略在具有非常放热的键合步骤的共价抑制剂的研究中特别重要。总的来说,我们的结果证明了了解化学反应步骤在设计酪氨酸激酶选择性共价抑制剂中的重要性。
更新日期:2022-08-31
中文翻译:

探索化学反应在酪氨酸激酶抑制剂选择性中的作用
许多疾病都与酪氨酸激酶有关,酪氨酸激酶通过信号转导级联激活许多蛋白质。这些酪氨酸激酶相似的 ATP 结合袋使得设计选择性共价抑制剂变得极其困难。本研究探讨了化学反应步骤对商业化抑制剂 acalabrutinib 相对于布鲁顿酪氨酸激酶 (BTK) 和白细胞介素 2 诱导型 T 细胞激酶 (ITK) 的选择性的贡献。两种激酶的从头计算和经验价键 (EVB) 模拟表明,最有利的反应路径涉及 acalabrutinib 的 2-丁酰胺反应基团的水辅助机制。根据我们计算的自由能曲线和动力学模拟,BTK 与 acalabrutinib 发生反应的势垒比 ITK 低得多。这种差异是由于活性位点的微环境造成的,对几种商业化抑制剂的特异性决定因素的基于序列的分析进一步支持了这一点。我们的研究涉及一种直接模拟 IC50 和灭活效率k eff的新方法,而不是使用标准公式。这种新策略在具有非常放热的键合步骤的共价抑制剂的研究中特别重要。总的来说,我们的结果证明了了解化学反应步骤在设计酪氨酸激酶选择性共价抑制剂中的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号