Synthesis ( IF 2.2 ) Pub Date : 2022-08-31 , DOI: 10.1055/a-1900-8895 Biprajit Paul , Hrishikesh Paul , Indranil Chatterjee
|
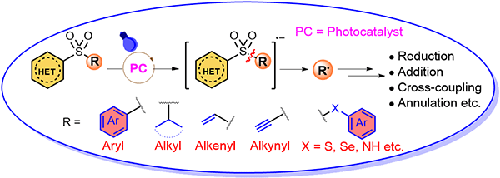
In recent times, desulfonylative radical-cross-coupling (RCC) has come to the forefront in synthetic organic, bio, and material chemistry as a powerful strategy to form C–C and C–heteroatom bonds. Diverse functionalization through metal- and photoredox-catalyzed desulfonylation reactions has attracted the scientific community due to the mild reaction conditions, wide functional group tolerance, and excellent synthetic efficacy. In this review, we have highlighted photoredox-mediated desulfonylation reactions developed since 2000. This review will summarize the newly reported methodologies, with particular emphasis on their mechanistic aspects and selectivity issues which have paved a new way towards sustainable C–C and C–X (X = H or heteroatom) bond formation.
1 Introduction
2 Photoredox-Catalyzed C–C Bond Formation
2.1 Aryl Sulfones as Radical Precursor
2.2 Reactions of Allyl Sulfones
3 Photoredox-Catalyzed C–Heteroatom Bond Formation
4 Conclusion
中文翻译:

光氧化还原介导的脱磺基自由基反应:形成 C-C 和 C-杂原子键的绝佳方法
近年来,脱磺基自由基交叉偶联 (RCC) 作为一种形成 C-C 和 C-杂原子键的强大策略已成为合成有机、生物和材料化学的前沿。通过金属和光氧化还原催化的脱磺酰化反应进行的多样化功能化由于其温和的反应条件、广泛的官能团耐受性和优异的合成功效而引起了科学界的关注。在这篇综述中,我们重点介绍了自 2000 年以来开发的光氧化还原介导的脱磺酰化反应。这篇综述将总结新报道的方法,特别强调它们的机制方面和选择性问题,这些问题为可持续的 C-C 和 C-X 铺平了新的道路(X = H 或杂原子) 键的形成。
1 简介
2 光氧化还原催化的 C-C 键形成
2.1 芳基砜作为自由基前体
2.2 烯丙基砜的反应
3 光氧化还原催化的 C-杂原子键形成
4。结论































 京公网安备 11010802027423号
京公网安备 11010802027423号