当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atroposelective Electrophilic Sulfenylation of N-Aryl Aminoquinone Derivatives Catalyzed by Chiral SPINOL-Derived Sulfide
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-08-30 , DOI: 10.1002/anie.202211782 Deng Zhu 1 , Lu Yu 2, 3 , Hui-Yun Luo 1 , Xiao-Song Xue 3, 4 , Zhi-Min Chen 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-08-30 , DOI: 10.1002/anie.202211782 Deng Zhu 1 , Lu Yu 2, 3 , Hui-Yun Luo 1 , Xiao-Song Xue 3, 4 , Zhi-Min Chen 1
Affiliation
|
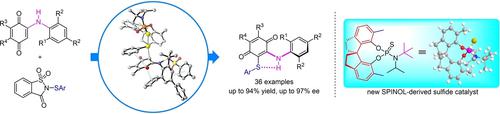
Atroposelective electrophilic sulfenylation of N-aryl aminoquinone derivatives catalyzed by a new chiral SPINOL-derived sulfide has been achieved. Axially chiral sulfur-containing diarylamine derivatives were obtained in moderate to excellent yields with moderate to excellent enantioselectivities. The intramolecular N−H⋅⋅⋅S hydrogen bond is a key parameter for the stability of the C−N axis. DFT calculations revealed the origin of atroposelectivity.
中文翻译:

手性 SPINOL 衍生硫化物催化 N-芳基氨基醌衍生物的阻转选择性亲电磺酰化
已经实现了由新型手性 SPINOL 衍生的硫化物催化的N-芳基氨基醌衍生物的阻转选择性亲电亚磺酰化。轴向手性含硫二芳基胺衍生物以中等至优异的产率获得,具有中等至优异的对映选择性。分子内 N−H····S 氢键是 C−N 轴稳定性的关键参数。DFT 计算揭示了回旋选择性的起源。
更新日期:2022-08-30
中文翻译:

手性 SPINOL 衍生硫化物催化 N-芳基氨基醌衍生物的阻转选择性亲电磺酰化
已经实现了由新型手性 SPINOL 衍生的硫化物催化的N-芳基氨基醌衍生物的阻转选择性亲电亚磺酰化。轴向手性含硫二芳基胺衍生物以中等至优异的产率获得,具有中等至优异的对映选择性。分子内 N−H····S 氢键是 C−N 轴稳定性的关键参数。DFT 计算揭示了回旋选择性的起源。































 京公网安备 11010802027423号
京公网安备 11010802027423号