当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Interchain Crosslinking by Alkyl Dihalides on the Electrochemical Performance of Nanoscale Polypyrrole Films
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-08-29 , DOI: 10.1021/acs.chemmater.2c02225 Ryan C. Gettler 1 , Naresh Alaal 2 , Kurt R. Brorsen 2 , Matthias J. Young 1, 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-08-29 , DOI: 10.1021/acs.chemmater.2c02225 Ryan C. Gettler 1 , Naresh Alaal 2 , Kurt R. Brorsen 2 , Matthias J. Young 1, 2
Affiliation
|
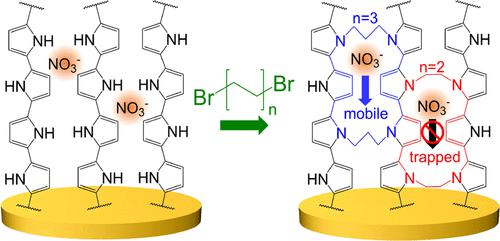
Nanoscale films of redox-active amine polymers such as polypyrrole (Ppy) are of interest for aqueous energy storage, water treatment, and chemical sensors. Unfortunately, the electrochemical properties of Ppy are constrained by the local material structures that form during typical synthesis. In this study, we examine how crosslinking Ppy postsynthesis with short-chain bifunctional alkyl-halide crosslinkers influences the charge storage properties of Ppy. Specifically, we employ dibromoethane (EtBr2), dibromopropane (PrBr2), and dibromobutane (BuBr2) crosslinkers to link amines from adjacent Ppy polymer chains in nanoscale Ppy films formed by electrodeposition. We study the electrochemical performance of the resulting structures using an electrochemical quartz crystal microbalance complemented by density-functional theory studies. We identify that the shortest (ethyl) crosslinker sterically traps free anions from the electrolyte within the Ppy structure. These trapped anions lead to a qualitative shift in the electrochemical mechanism from anion-insertion to cation-insertion behavior. We identify that the propyl crosslinker, with just one carbon more than ethyl, allows for more rapid anion motion than intrinsic Ppy, accessing electrochemical capacities up to 60% higher than that with no crosslinker. These results reveal the strong impact of the local molecular structure on the electrochemical properties of redox-active polymers and demonstrate the use of short-chain bifunctional crosslinkers to control their qualitative electrochemical response.
中文翻译:

烷基二卤化物链间交联对纳米聚吡咯薄膜电化学性能的影响
聚吡咯 (Ppy) 等氧化还原活性胺聚合物的纳米级薄膜对于水性能量存储、水处理和化学传感器具有重要意义。不幸的是,Ppy 的电化学性质受到典型合成过程中形成的局部材料结构的限制。在本研究中,我们研究了用短链双功能烷基卤化物交联剂交联 Ppy 后合成如何影响 Ppy 的电荷存储特性。具体来说,我们采用二溴乙烷 (EtBr 2 )、二溴丙烷 (PrBr 2 ) 和二溴丁烷 (BuBr 2) 交联剂将胺从相邻的 Ppy 聚合物链中连接到通过电沉积形成的纳米级 Ppy 薄膜中。我们使用电化学石英晶体微天平辅以密度泛函理论研究来研究所得结构的电化学性能。我们发现最短的(乙基)交联剂在空间上从 Ppy 结构内的电解质中捕获游离阴离子。这些被捕获的阴离子导致电化学机制从阴离子插入到阳离子插入行为发生质的转变。我们发现,仅比乙基多一个碳的丙基交联剂允许比固有 Ppy 更快的阴离子运动,其电化学容量比没有交联剂的高 60%。
更新日期:2022-08-29
中文翻译:

烷基二卤化物链间交联对纳米聚吡咯薄膜电化学性能的影响
聚吡咯 (Ppy) 等氧化还原活性胺聚合物的纳米级薄膜对于水性能量存储、水处理和化学传感器具有重要意义。不幸的是,Ppy 的电化学性质受到典型合成过程中形成的局部材料结构的限制。在本研究中,我们研究了用短链双功能烷基卤化物交联剂交联 Ppy 后合成如何影响 Ppy 的电荷存储特性。具体来说,我们采用二溴乙烷 (EtBr 2 )、二溴丙烷 (PrBr 2 ) 和二溴丁烷 (BuBr 2) 交联剂将胺从相邻的 Ppy 聚合物链中连接到通过电沉积形成的纳米级 Ppy 薄膜中。我们使用电化学石英晶体微天平辅以密度泛函理论研究来研究所得结构的电化学性能。我们发现最短的(乙基)交联剂在空间上从 Ppy 结构内的电解质中捕获游离阴离子。这些被捕获的阴离子导致电化学机制从阴离子插入到阳离子插入行为发生质的转变。我们发现,仅比乙基多一个碳的丙基交联剂允许比固有 Ppy 更快的阴离子运动,其电化学容量比没有交联剂的高 60%。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号