Journal of Luminescence ( IF 3.3 ) Pub Date : 2022-08-28 , DOI: 10.1016/j.jlumin.2022.119258 Priyanka Kumari , Kamakhya Prakash Misra , Susruta Samanta , Ashok Rao , Atul Bandyopadhyay , Saikat Chattopadhyay
|
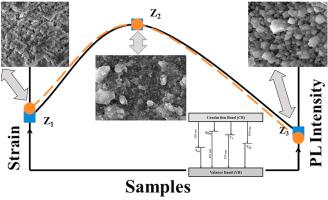
ZnS 和铈 (Ce) 掺杂的 ZnS 的三维 (3-D) 量子结构 (QSs) 是通过化学上负担得起的溶胶-凝胶工艺合成的。探讨了 Ce 掺杂以及由此引起的微应变对结构、形态和光学特性的影响。XRD证实了单相闪锌矿ZnS的形成。对应于最高强度 XRD 峰 (111) 的估计平均晶体尺寸在 1.65-4.65 nm 范围内变化,这与 ZnS 的玻尔半径相当。由于 Ce 和 Zn 的尺寸不匹配,在 ZnS 的基质中发现了微应变和空位。热力学计算验证了由于 Ce 掺杂引起的晶格参数的膨胀和收缩。FTIR 光谱证实了与 Zn 和 S 相关的不同官能团的存在。在 420、461、509和560 nm分别与间隙硫、锌间隙、硫空位和锌空位等缺陷态有关。掺杂 ZnS 在 600 nm 处另一个发射峰的上升是由于Ce 3+离子中的5d → 4f能级跃迁。演化的微应变分布、PL 强度和能带隙变化在掺杂浓度方面彼此相似。显微图像证实了随着掺杂浓度的增加,结构转变为立方体形 ZnS QSs。EDX 和 XPS 支持元素分析以及可用元素(如 Zn、S 和 Ce)的氧化态。
,ZnS 和铈 (Ce) 掺杂的 ZnS 的三维 (3-D) 量子结构 (QSs) 是通过化学上负担得起的溶胶-凝胶工艺合成的。探讨了 Ce 掺杂以及由此引起的微应变对结构、形态和光学特性的影响。XRD证实了单相闪锌矿ZnS的形成。对应于最高强度 XRD 峰 (111) 的估计平均晶体尺寸在 1.65-4.65 nm 范围内变化,这与 ZnS 的玻尔半径相当。由于 Ce 和 Zn 的尺寸不匹配,在 ZnS 的基质中发现了微应变和空位。热力学计算验证了由于 Ce 掺杂引起的晶格参数的膨胀和收缩。FTIR 光谱证实了与 Zn 和 S 相关的不同官能团的存在。在 420、461、509和560 nm分别与间隙硫、锌间隙、硫空位和锌空位等缺陷态有关。掺杂 ZnS 在 600 nm 处另一个发射峰的上升是由于Ce 3+离子中的5d → 4f能级跃迁。演化的微应变分布、PL 强度和能带隙变化在掺杂浓度方面彼此相似。显微图像证实了随着掺杂浓度的增加,结构转变为立方体形 ZnS QSs。EDX 和 XPS 支持元素分析以及可用元素(如 Zn、S 和 Ce)的氧化态。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号