当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H2O Adsorption/Dissociation and H2 Generation by the Reaction of H2O with Al2O3 Materials: A First-Principles Investigation
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-09-08 00:00:00 , DOI: 10.1021/acs.jpcc.6b07191
Yu-Huan Lu,Shiuan-Yau Wu,Hsin-Tsung Chen
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-09-08 00:00:00 , DOI: 10.1021/acs.jpcc.6b07191
Yu-Huan Lu,Shiuan-Yau Wu,Hsin-Tsung Chen
|
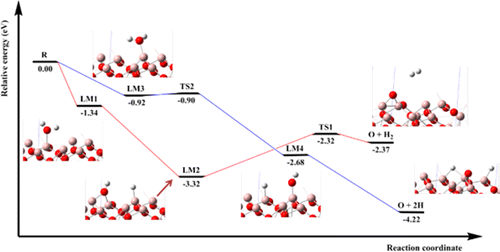
The microscopic reaction mechanisms for the water adsorption/dissociation and hydrogen generation processes on the α-Al2O3(0001) surface are clarified by using spin-polarized density functional theory with the projected augmented wave approach. The adsorptions of OH, O, and H species are also examined. Calculations show that the H2O, OH, O, and H species prefer to adsorb at the Al(II)-top, Al(I, II)-top, Al(I, II)-bridge, and Al(II)-top sites with adsorption energies of −1.34, −5.91, −8.22, and −3.14 eV on the Al-terminated surface, whereas those are Al-top, Al-top, Al-top, and O-top sites with adsorption energies of −1.11, −2.79, −2.00, and −2.23 eV for the Al, O-terminated surface. Geometries of the molecular adsorbed intermediates, transition states, and the hydroxylated products as well as the energetic reaction routes are fully elucidated. Hydrogen generation and full dissociation of water are found to occur on the Al-terminated surface with overall exothermicities of 2.37 and 4.22 eV, whereas only the production of coadsorbed H(ads) + OH(ads) is observed on the Al, O-terminated surface with an overall exothermicity of 1.06–1.64 eV. In addition, the local density of states and Bader charge calculations are carried out to study the interaction between the adsorbate and surface along the reaction.
中文翻译:

ħ 2 ö的吸附/离解和H 2生成由H的反应2 ö用Al 2 ö 3材料:第一原理调查
为所述α-Al对水的吸附/离解和氢产生过程中的微观反应机理2 ö 3(0001)面是通过使用与所述投影缀波方法自旋极化密度泛函理论阐明。还检查了OH,O和H物质的吸附。计算表明,H 2O,OH,O和H物质更喜欢在具有吸附能的Al(II)顶部,Al(I,II)顶部,Al(I,II)桥和Al(II)顶部位置进行吸附在终止于Al的表面上的-1.34,-5.91,-8.22和-3.14 eV分别为Al-top,Al-top,Al-top和O-top位置,其吸附能为-1.11,-2.79对于铝,O端接表面,分别为-2.00和-2.23 eV。充分阐明了分子吸附的中间体的几何形状,过渡态和羟基化产物以及高能反应路线。发现氢的生成和水的完全分解在铝终止的表面上发生,总放热度为2.37和4.22 eV,而仅产生共吸附的H (ads) + OH (ads)在铝,O端表面观察到总放热为1.06-1.64 eV。另外,进行状态的局部密度和巴德电荷计算以研究沿着反应的被吸附物和表面之间的相互作用。
更新日期:2016-09-08
中文翻译:

ħ 2 ö的吸附/离解和H 2生成由H的反应2 ö用Al 2 ö 3材料:第一原理调查
为所述α-Al对水的吸附/离解和氢产生过程中的微观反应机理2 ö 3(0001)面是通过使用与所述投影缀波方法自旋极化密度泛函理论阐明。还检查了OH,O和H物质的吸附。计算表明,H 2O,OH,O和H物质更喜欢在具有吸附能的Al(II)顶部,Al(I,II)顶部,Al(I,II)桥和Al(II)顶部位置进行吸附在终止于Al的表面上的-1.34,-5.91,-8.22和-3.14 eV分别为Al-top,Al-top,Al-top和O-top位置,其吸附能为-1.11,-2.79对于铝,O端接表面,分别为-2.00和-2.23 eV。充分阐明了分子吸附的中间体的几何形状,过渡态和羟基化产物以及高能反应路线。发现氢的生成和水的完全分解在铝终止的表面上发生,总放热度为2.37和4.22 eV,而仅产生共吸附的H (ads) + OH (ads)在铝,O端表面观察到总放热为1.06-1.64 eV。另外,进行状态的局部密度和巴德电荷计算以研究沿着反应的被吸附物和表面之间的相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号