Science of the Total Environment ( IF 8.2 ) Pub Date : 2022-08-28 , DOI: 10.1016/j.scitotenv.2022.158345 Haochen Zhang 1 , Zhuoyu Li 2 , Xiaoqun Zhou 1 , Xiaohui Lu 1 , Haiteng Gu 1 , Jun Ma 1
|
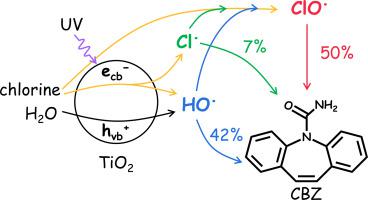
The UV/chlorine (UC) system is a homogeneous advanced oxidation process with increasing attention in water decontamination. The addition of TiO2 is a newly found strategy to enhance the generation of hydroxyl radical (HO•) and chlorine radical (Cl•) in the UC system. However, the crucial role of chlorine oxide radical (ClO•, generated by the reactions of HO• and Cl• with chlorine) on pollutant degradation, has not been noticed in UV/chlorine/TiO2 (UCT), the heterogeneous photocatalytic system for chlorine activation. Herein, the role of ClO• in UCT was clarified through quenching experiments combined with model simulations during carbamazepine degradation. Tert-butyl alcohol completely inhibited while bicarbonate only partly suppressed carbamazepine degradation in UCT, indicating the important role of ClO•. The second-order reaction rate constant between ClO• and carbamazepine (kClO•,carbamazepine) was fitted to be (1.21 ± 0.08) × 107 M−1 s−1 by the kinetic model, which avoided the influence of carbonate radical (CO3•−), whose contribution couldn't be excluded during kClO•,carbamazepine determination in commonly used competitive kinetic methods with bicarbonate. With the obtained kClO•,carbamazepine, model simulation suggested that ClO• contributed about 50 % to carbamazepine degradation in UCT, and its concentration was less affected under varied conditions (solution pH, chlorine, bicarbonate, and chloride concentration) to keep an efficient carbamazepine degradation. On the contrary, pollutant degradation dominated by HO• in UCT was largely inhibited with the increase of pH, chlorine, and bicarbonate concentration. In addition to the promotion of degradation efficiency, less disinfection byproducts and lower energy requirement were found in UCT compared with UC. Furthermore, UCT could maintain satisfactory degradation efficiency and energy saving in ground water and surface water samples. Results of this study unraveled the crucial role of ClO• for pollutant degradation in UCT, and showed bright prospects and great potentials of the system in water treatment.
中文翻译:

深入了解紫外/氯/二氧化钛对卡马西平降解的性能:氯氧化物自由基 (ClO•) 的关键作用
紫外/氯 (UC) 系统是一种均相高级氧化工艺,在水净化方面越来越受到关注。添加 TiO 2是一种新发现的策略,可增强UC 系统中羟基自由基 (HO • ) 和氯自由基 (Cl • ) 的生成。然而,氯氧化物自由基(ClO •,由H2O •和Cl •与氯反应产生)对污染物降解的关键作用,在UV/氯/TiO 2 (UCT) 多相光催化系统中尚未被注意到。氯活化。在此,二氧化氯的作用•通过淬灭实验结合卡马西平降解过程中的模型模拟,阐明了 UCT 中的含量。UCT中叔丁醇完全抑制,而碳酸氢盐仅部分抑制卡马西平降解,表明ClO •的重要作用。动力学模型拟合ClO •与卡马西平的二级反应速率常数( k ClO•,carbamazepine )为(1.21 ± 0.08) × 10 7 M -1 s -1,避免了碳酸盐自由基的影响( CO 3 •− ),在k ClO•,carbamazepine期间不能排除其贡献在常用的竞争动力学方法中用碳酸氢盐进行测定。使用得到的kClO •,卡马西平,模型模拟表明,ClO •对 UCT 中卡马西平降解的贡献约为 50 %,并且其浓度在不同条件(溶液 pH、氯、碳酸氢盐和氯化物浓度)下影响较小,以保持高效卡马西平降解。相反,以 H2O 为主的污染物降解•随着 pH 值、氯和碳酸氢盐浓度的增加,UCT 的含量受到很大抑制。除了提高降解效率外,与 UC 相比,UCT 中发现的消毒副产物更少,能量需求更低。此外,UCT 可以在地下水和地表水样品中保持令人满意的降解效率和节能效果。本研究结果揭示了ClO •在UCT中对污染物降解的关键作用,并展示了该系统在水处理中的广阔前景和巨大潜力。






































 京公网安备 11010802027423号
京公网安备 11010802027423号