当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of (R)-(2-Chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyridin-2-yl)-4-methyl-6,7-dihydro-1H-imidazo[4,5-c]pyridin-5(4H)-yl)methanone (JNJ 54166060), a Small Molecule Antagonist of the P2X7 receptor
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-09-08 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00989 Devin M. Swanson 1 , Brad M. Savall 1 , Kevin J. Coe 1 , Freddy Schoetens 1 , Tatiana Koudriakova 1 , Judith Skaptason 1 , Jessica Wall 1 , Jason Rech 1 , Xiahou Deng 1 , Meri De Angelis 2 , Anita Everson 1 , Brian Lord 1 , Qi Wang 1 , Hong Ao 1 , Brian Scott 1 , Kia Sepassi 1 , Timothy W. Lovenberg 1 , Nicholas I. Carruthers 1 , Anindya Bhattacharya 1 , Michael A. Letavic 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-09-08 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00989 Devin M. Swanson 1 , Brad M. Savall 1 , Kevin J. Coe 1 , Freddy Schoetens 1 , Tatiana Koudriakova 1 , Judith Skaptason 1 , Jessica Wall 1 , Jason Rech 1 , Xiahou Deng 1 , Meri De Angelis 2 , Anita Everson 1 , Brian Lord 1 , Qi Wang 1 , Hong Ao 1 , Brian Scott 1 , Kia Sepassi 1 , Timothy W. Lovenberg 1 , Nicholas I. Carruthers 1 , Anindya Bhattacharya 1 , Michael A. Letavic 1
Affiliation
|
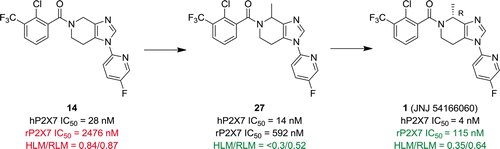
The synthesis and SAR of a series of 4,5,6,7-tetrahydro-imidazo[4,5-c]pyridine P2X7 antagonists are described. Addressing P2X7 affinity and liver microsomal stability issues encountered with this template afforded methyl substituted 4,5,6,7-tetrahydro-imidazo[4,5-c]pyridines ultimately leading to the identification of 1 (JNJ 54166060). 1 is a potent P2X7 antagonist with an ED50 = 2.3 mg/kg in rats, high oral bioavailability and low-moderate clearance in preclinical species, acceptable safety margins in rats, and a predicted human dose of 120 mg of QD. Additionally, 1 possesses a unique CYP profile and was found to be a regioselective inhibitor of midazolam CYP3A metabolism.
中文翻译:

(R)-(2-氯-3-(三氟甲基)苯基)(1-(5-氟吡啶-2-基)-4-甲基-6,7-二氢-1 H-咪唑[4,5- c ] pyridin-5(4 H)-yl)methanone(JNJ 54166060),P2X7受体的小分子拮抗剂
描述了一系列4,5,6,7-四氢咪唑并[4,5- c ]吡啶P2X7拮抗剂的合成和SAR 。解决此模板遇到的P2X7亲和力和肝微粒体稳定性问题,提供了甲基取代的4,5,6,7-四氢咪唑并[4,5- c ]吡啶,最终导致1的鉴定(JNJ 54166060)。1是一种有效的P2X7拮抗剂,在大鼠中的ED 50 = 2.3 mg / kg,在临床前物种中具有较高的口服生物利用度和低中度清除率,在大鼠中具有可接受的安全范围,并且预计的人类剂量为120 mg QD。此外,1具有独特的CYP谱,被发现是咪达唑仑CYP3A代谢的区域选择性抑制剂。
更新日期:2016-09-08
中文翻译:

(R)-(2-氯-3-(三氟甲基)苯基)(1-(5-氟吡啶-2-基)-4-甲基-6,7-二氢-1 H-咪唑[4,5- c ] pyridin-5(4 H)-yl)methanone(JNJ 54166060),P2X7受体的小分子拮抗剂
描述了一系列4,5,6,7-四氢咪唑并[4,5- c ]吡啶P2X7拮抗剂的合成和SAR 。解决此模板遇到的P2X7亲和力和肝微粒体稳定性问题,提供了甲基取代的4,5,6,7-四氢咪唑并[4,5- c ]吡啶,最终导致1的鉴定(JNJ 54166060)。1是一种有效的P2X7拮抗剂,在大鼠中的ED 50 = 2.3 mg / kg,在临床前物种中具有较高的口服生物利用度和低中度清除率,在大鼠中具有可接受的安全范围,并且预计的人类剂量为120 mg QD。此外,1具有独特的CYP谱,被发现是咪达唑仑CYP3A代谢的区域选择性抑制剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号