当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intracellular miRNA Imaging Based on a Self-Powered and Self-Feedback Entropy-Driven Catalyst–DNAzyme Circuit
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-08-26 , DOI: 10.1021/acsami.2c11923 Chao Xing 1 , Qitian Lin 2 , Xue Gao 1 , Ting Cao 1 , Jing Chen 1 , Jialing Liu 1 , Yuhong Lin 3 , Jun Wang 1 , Chunhua Lu 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-08-26 , DOI: 10.1021/acsami.2c11923 Chao Xing 1 , Qitian Lin 2 , Xue Gao 1 , Ting Cao 1 , Jing Chen 1 , Jialing Liu 1 , Yuhong Lin 3 , Jun Wang 1 , Chunhua Lu 2
Affiliation
|
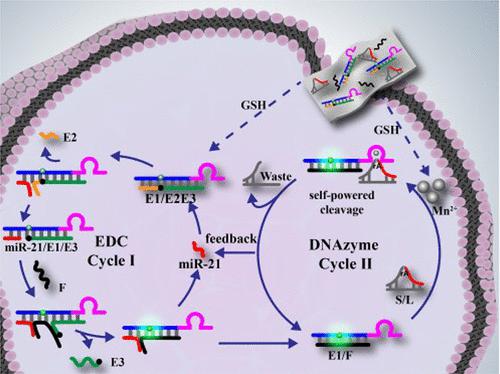
DNAzyme-based signal amplification circuits promote the advances in low-abundant miRNA imaging in living cells. However, due to the insufficient cofactor in living cells and unsustainable target utilization, self-powered and self-feedback DNAzyme amplification circuits have rarely been achieved. Here, a MnO2 nanosheet-mediated self-powered and self-feedback entropy-driven catalyst (EDC)–DNAzyme nanoprobe (MnPFEDz) was demonstrated for sensitive imaging of intracellular microRNA (miRNA). In this strategy, MnPFEDz was formed by adsorbing EDC modules and substrate probes on MnO2 nanosheets. The MnO2 nanosheets acted not only as glutathione (GSH)-responsive nanocarriers for efficient delivery of DNA probes but also as a DNAzyme cofactor supplier to power the DNAzyme biocatalysis and promote signal transduction in a feedback way. When entering the cells, GSH could decompose MnO2 nanosheets to generate numerous Mn2+ ion cofactors, leading to the release of DNA probes. Subsequently, the target miRNA initiated EDC cycles to generate amplified fluorescence signals and exposed the complete DNAzyme. Meanwhile, each of the exposed DNAzyme then cleaved the substrate probes with the help of Mn2+ ion cofactors and released a new trigger analogue for the next round of EDC cycles, initiating additional fluorescence signals in a feedback way. As a multiple signal amplification strategy, the MnPFEDz nanoprobe facilitated the effective detection of intracellular molecules with enhanced sensitivity and provided a versatile strategy for the construction of self-powered and self-feedback DNA circuits in living cells.
中文翻译:

基于自供电和自反馈熵驱动催化剂-脱氧核酶电路的细胞内 miRNA 成像
基于 DNAzyme 的信号放大电路促进了活细胞中低丰度 miRNA 成像的进展。然而,由于活细胞中辅因子不足和靶点利用不可持续,很少实现自供电和自反馈脱氧核糖核酸酶扩增电路。在这里,MnO 2纳米片介导的自供电和自反馈熵驱动催化剂 (EDC)-DNAzyme 纳米探针 (MnPFEDz) 被证明可用于细胞内 microRNA (miRNA) 的灵敏成像。在该策略中,MnPFEDz 是通过在 MnO 2纳米片上吸附 EDC 模块和衬底探针形成的。二氧化锰纳米片不仅可作为谷胱甘肽 (GSH) 响应的纳米载体,以有效递送 DNA 探针,而且还可作为 DNAzyme 辅因子供应商,为 DNAzyme 生物催化提供动力并以反馈方式促进信号转导。GSH进入细胞后可分解MnO 2纳米片产生大量Mn 2+离子辅因子,从而释放DNA探针。随后,目标 miRNA 启动 EDC 循环以产生放大的荧光信号并暴露完整的 DNAzyme。同时,每个暴露的DNAzyme随后在Mn 2+的帮助下切割底物探针离子辅助因子,并为下一轮 EDC 循环发布了新的触发类似物,以反馈方式启动额外的荧光信号。作为一种多信号放大策略,MnPFEDz 纳米探针有助于有效检测细胞内分子并提高灵敏度,并为在活细胞中构建自供电和自反馈 DNA 电路提供了一种通用策略。
更新日期:2022-08-26
中文翻译:

基于自供电和自反馈熵驱动催化剂-脱氧核酶电路的细胞内 miRNA 成像
基于 DNAzyme 的信号放大电路促进了活细胞中低丰度 miRNA 成像的进展。然而,由于活细胞中辅因子不足和靶点利用不可持续,很少实现自供电和自反馈脱氧核糖核酸酶扩增电路。在这里,MnO 2纳米片介导的自供电和自反馈熵驱动催化剂 (EDC)-DNAzyme 纳米探针 (MnPFEDz) 被证明可用于细胞内 microRNA (miRNA) 的灵敏成像。在该策略中,MnPFEDz 是通过在 MnO 2纳米片上吸附 EDC 模块和衬底探针形成的。二氧化锰纳米片不仅可作为谷胱甘肽 (GSH) 响应的纳米载体,以有效递送 DNA 探针,而且还可作为 DNAzyme 辅因子供应商,为 DNAzyme 生物催化提供动力并以反馈方式促进信号转导。GSH进入细胞后可分解MnO 2纳米片产生大量Mn 2+离子辅因子,从而释放DNA探针。随后,目标 miRNA 启动 EDC 循环以产生放大的荧光信号并暴露完整的 DNAzyme。同时,每个暴露的DNAzyme随后在Mn 2+的帮助下切割底物探针离子辅助因子,并为下一轮 EDC 循环发布了新的触发类似物,以反馈方式启动额外的荧光信号。作为一种多信号放大策略,MnPFEDz 纳米探针有助于有效检测细胞内分子并提高灵敏度,并为在活细胞中构建自供电和自反馈 DNA 电路提供了一种通用策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号