当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
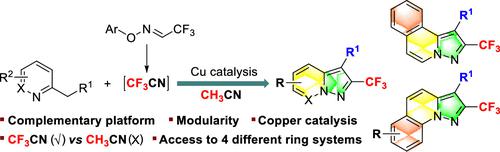
Modular Access to 2-(Trifluoromethyl)pyrazolo[1,5-a]pyridines and Their Benzo Analogues through a Copper(I)-Catalyzed Radical Annulation
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-25 , DOI: 10.1021/acs.orglett.2c02500 Zhenhui Wang 1 , Xiaofeng Li 1 , Jie Qiu 1 , Wei Li 1 , Hengyuan Li 1 , Zhiqiang Weng 2 , Huaifeng Li 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-25 , DOI: 10.1021/acs.orglett.2c02500 Zhenhui Wang 1 , Xiaofeng Li 1 , Jie Qiu 1 , Wei Li 1 , Hengyuan Li 1 , Zhiqiang Weng 2 , Huaifeng Li 1
Affiliation
|
A mechanistically distinctive copper-catalyzed radical annulation to valuable 2-(trifluoromethyl)pyrazolo[1,5-a]pyridines and their benzo analogues has been described for the first time. Notably, the newly developed complementary process allows the synthesis of 4- or 6-substituted target molecular entities as a single product, which was previously challenging to access by existing methods. The utility of this process is further demonstrated by the facile construction of four different ring systems, a gram-scale synthesis, and the late-stage functionalization of bioactive molecules.
中文翻译:

通过铜 (I) 催化自由基环化对 2-(三氟甲基) 吡唑并[1,5-a] 吡啶及其苯并类似物进行模块化访问
首次描述了一种机制独特的铜催化自由基环化对有价值的 2-(三氟甲基)吡唑并[1,5- a ] 吡啶及其苯并类似物的反应。值得注意的是,新开发的补充工艺允许将 4 或 6 取代的目标分子实体作为单一产品合成,这在以前很难通过现有方法获得。该过程的实用性通过四种不同环系统的简易构建、克级合成和生物活性分子的后期功能化得到进一步证明。
更新日期:2022-08-25
中文翻译:

通过铜 (I) 催化自由基环化对 2-(三氟甲基) 吡唑并[1,5-a] 吡啶及其苯并类似物进行模块化访问
首次描述了一种机制独特的铜催化自由基环化对有价值的 2-(三氟甲基)吡唑并[1,5- a ] 吡啶及其苯并类似物的反应。值得注意的是,新开发的补充工艺允许将 4 或 6 取代的目标分子实体作为单一产品合成,这在以前很难通过现有方法获得。该过程的实用性通过四种不同环系统的简易构建、克级合成和生物活性分子的后期功能化得到进一步证明。














































 京公网安备 11010802027423号
京公网安备 11010802027423号