当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Locked DNAzyme Platform for In Vitro and In Vivo Discrimination of Cancer Cells
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-08-24 , DOI: 10.1021/acs.analchem.2c02788 Xing Huang 1 , Yanfei Zhang 2 , Jun Chen 3 , Lang Zhang 2 , Yuzhi Xu 4 , Wen Yin 2 , Yakun Shi 1 , Si-Yang Liu 1 , Xiaoyong Zou 2 , Zong Dai 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-08-24 , DOI: 10.1021/acs.analchem.2c02788 Xing Huang 1 , Yanfei Zhang 2 , Jun Chen 3 , Lang Zhang 2 , Yuzhi Xu 4 , Wen Yin 2 , Yakun Shi 1 , Si-Yang Liu 1 , Xiaoyong Zou 2 , Zong Dai 1
Affiliation
|
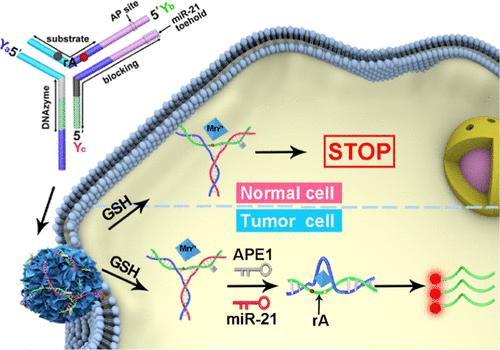
Imaging of tumor-associated microRNAs (miRNAs) can provide abundant information for cancer diagnosis, whereas the occurrence of trace amounts of miRNAs in normal cells inevitably causes an undesired false-positive signal in the discrimination of cancer cells during miRNA imaging. In this study, we propose a dual-locked (D-locked) platform consisting of the enzyme/miRNA-D-locked DNAzyme sensor and the honeycomb MnO2 nanosponge (hMNS) nanocarrier for highly specific cancer cell imaging. For a proof-of-concept demonstration, apurinic/apyrimidinic endonuclease 1 (APE1) and miR-21 were chosen as key models. The hMNS nanocarrier can efficiently release the D-locked DNAzyme sensor in living cells due to the decomposition of hMNS by glutathione, which can also supply Mn2+ for DNAzyme cleavage. Ascribing to the smart design of the D-locked DNAzyme sensor, the fluorescence signal can only be generated by the synergistic response of APE1 and miR-21 that are overexpressed in cancer cells. Compared with the miRNA single-locked DNAzyme sensor and the small-molecule (ATP)/miRNA D-locked DNAzyme sensor, the proposed enzyme (APE1)/miRNA D-locked DNAzyme sensor exhibited 2.6-fold and 2.4-fold higher discrimination ratio (Fcancer/Fnormal) for cancer cell discrimination, respectively. Owing to the superior performance, the D-locked strategy can selectively generate a fluorescence signal in cancer cells, facilitating accurate discrimination of cancer both in vitro and in vivo. Furthermore, this D-locked platform is easily adaptable toward other target molecules by redesigning the DNA sequences. The outstanding performance and expansibility of this D-locked platform holds promising prospects for cancer diagnosis and related biomedical applications.
中文翻译:

用于癌细胞体外和体内鉴别的双锁定 DNAzyme 平台
肿瘤相关微小RNA(miRNA)的成像可以为癌症诊断提供丰富的信息,而正常细胞中微量miRNA的出现不可避免地会在miRNA成像过程中对癌细胞的鉴别产生不希望的假阳性信号。在这项研究中,我们提出了一种由酶/miRNA-D 锁定 DNAzyme 传感器和蜂窝状 MnO 2纳米海绵 (hMNS) 纳米载体组成的双锁定(D 锁定)平台,用于高度特异性的癌细胞成像。对于概念验证演示,选择无嘌呤/无嘧啶核酸内切酶 1 (APE1) 和 miR-21 作为关键模型。由于谷胱甘肽分解hMNS,hMNS纳米载体可以有效释放活细胞中的D-locked DNAzyme传感器,还可以提供Mn 2+用于 DNAzyme 切割。由于 D-locked DNAzyme 传感器的智能设计,荧光信号只能由在癌细胞中过表达的 APE1 和 miR-21 的协同反应产生。与 miRNA 单锁 DNAzyme 传感器和小分子 (ATP)/miRNA D-locked DNAzyme 传感器相比,所提出的酶 (APE1)/miRNA D-locked DNAzyme 传感器的识别率分别高出 2.6 倍和 2.4 倍。F cancer / F normal ) 分别用于癌细胞的鉴别。由于优异的性能,D-locked策略可以选择性地在癌细胞中产生荧光信号,有助于在体外和体内准确区分癌症. 此外,通过重新设计 DNA 序列,这个 D 锁定平台很容易适应其他靶分子。该D-locked平台的出色性能和可扩展性在癌症诊断和相关生物医学应用中具有广阔的前景。
更新日期:2022-08-24
中文翻译:

用于癌细胞体外和体内鉴别的双锁定 DNAzyme 平台
肿瘤相关微小RNA(miRNA)的成像可以为癌症诊断提供丰富的信息,而正常细胞中微量miRNA的出现不可避免地会在miRNA成像过程中对癌细胞的鉴别产生不希望的假阳性信号。在这项研究中,我们提出了一种由酶/miRNA-D 锁定 DNAzyme 传感器和蜂窝状 MnO 2纳米海绵 (hMNS) 纳米载体组成的双锁定(D 锁定)平台,用于高度特异性的癌细胞成像。对于概念验证演示,选择无嘌呤/无嘧啶核酸内切酶 1 (APE1) 和 miR-21 作为关键模型。由于谷胱甘肽分解hMNS,hMNS纳米载体可以有效释放活细胞中的D-locked DNAzyme传感器,还可以提供Mn 2+用于 DNAzyme 切割。由于 D-locked DNAzyme 传感器的智能设计,荧光信号只能由在癌细胞中过表达的 APE1 和 miR-21 的协同反应产生。与 miRNA 单锁 DNAzyme 传感器和小分子 (ATP)/miRNA D-locked DNAzyme 传感器相比,所提出的酶 (APE1)/miRNA D-locked DNAzyme 传感器的识别率分别高出 2.6 倍和 2.4 倍。F cancer / F normal ) 分别用于癌细胞的鉴别。由于优异的性能,D-locked策略可以选择性地在癌细胞中产生荧光信号,有助于在体外和体内准确区分癌症. 此外,通过重新设计 DNA 序列,这个 D 锁定平台很容易适应其他靶分子。该D-locked平台的出色性能和可扩展性在癌症诊断和相关生物医学应用中具有广阔的前景。















































 京公网安备 11010802027423号
京公网安备 11010802027423号