Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer
Advanced Science ( IF 14.3 ) Pub Date : 2022-08-18 , DOI: 10.1002/advs.202202914
Meng Wang 1, 2, 3, 4, 5, 6 , Min Wu 7, 8 , Xingang Liu 7 , Shiyi Shao 1, 2, 3, 4, 5, 6 , Junmin Huang 1, 2, 3, 4, 5, 6 , Bin Liu 7, 8 , Tingbo Liang 1, 2, 3, 4, 5, 6
Advanced Science ( IF 14.3 ) Pub Date : 2022-08-18 , DOI: 10.1002/advs.202202914
Meng Wang 1, 2, 3, 4, 5, 6 , Min Wu 7, 8 , Xingang Liu 7 , Shiyi Shao 1, 2, 3, 4, 5, 6 , Junmin Huang 1, 2, 3, 4, 5, 6 , Bin Liu 7, 8 , Tingbo Liang 1, 2, 3, 4, 5, 6
Affiliation
|
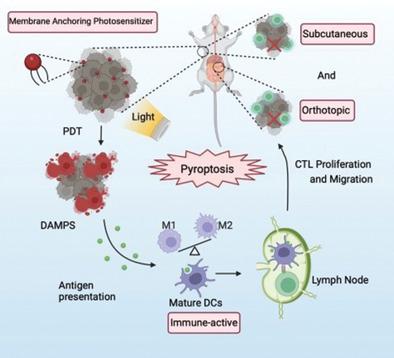
Immunotherapy, the most promising strategy of cancer treatment, has achieved promising outcomes, but its clinical efficacy in pancreatic cancer is limited mainly due to the complicated tumor immunosuppressive microenvironment. As a highly inflammatory form of immunogenic cell death (ICD), pyroptosis provides a great opportunity to alleviate immunosuppression and promote systemic immune responses in solid tumors. Herein, membrane-targeted photosensitizer TBD-3C with aggregation-induced emission (AIE) feature to trigger pyroptosis-aroused cancer immunotherapy via photodynamic therapy (PDT) is applied. The results reveal that pyroptotic cells induced by TBD-3C could stimulate M1-polarization of macrophages, cause maturation of dendritic cells (DCs), and activation of CD8+ cytotoxic T-lymphocytes (CTLs). Pyroptosis-aroused immunological responses could convert immunosuppressive “cold” tumor microenvironment (TME) to immunogenic “hot” TME, which not only inhibits primary pancreatic cancer growth but also attacks the distant tumor. This work establishes a platform with high biocompatibility for light-controlled antitumor immunity and solid tumor immunotherapy aroused by cell pyroptosis.
中文翻译:

焦亡重塑肿瘤微环境以增强膜锚定光敏剂驱动的胰腺癌免疫治疗
免疫治疗作为最有前景的癌症治疗策略,已取得了良好的效果,但其在胰腺癌中的临床疗效主要由于复杂的肿瘤免疫抑制微环境而受到限制。作为免疫原性细胞死亡(ICD)的一种高度炎症形式,焦亡为减轻实体瘤中的免疫抑制和促进全身免疫反应提供了绝佳的机会。在此,应用具有聚集诱导发射(AIE)功能的膜靶向光敏剂TBD-3C,通过光动力疗法(PDT)触发焦亡引发的癌症免疫治疗。结果表明,TBD-3C 诱导的焦亡细胞可以刺激巨噬细胞的 M1 极化,导致树突状细胞 (DC) 成熟,并激活 CD8 +细胞毒性 T 淋巴细胞 (CTL)。焦亡引起的免疫反应可以将免疫抑制性“冷”肿瘤微环境(TME)转变为免疫原性“热”TME,这不仅抑制原发性胰腺癌的生长,而且攻击远处的肿瘤。该工作为细胞焦亡引起的光控抗肿瘤免疫和实体瘤免疫治疗建立了一个具有高生物相容性的平台。
更新日期:2022-08-18
中文翻译:

焦亡重塑肿瘤微环境以增强膜锚定光敏剂驱动的胰腺癌免疫治疗
免疫治疗作为最有前景的癌症治疗策略,已取得了良好的效果,但其在胰腺癌中的临床疗效主要由于复杂的肿瘤免疫抑制微环境而受到限制。作为免疫原性细胞死亡(ICD)的一种高度炎症形式,焦亡为减轻实体瘤中的免疫抑制和促进全身免疫反应提供了绝佳的机会。在此,应用具有聚集诱导发射(AIE)功能的膜靶向光敏剂TBD-3C,通过光动力疗法(PDT)触发焦亡引发的癌症免疫治疗。结果表明,TBD-3C 诱导的焦亡细胞可以刺激巨噬细胞的 M1 极化,导致树突状细胞 (DC) 成熟,并激活 CD8 +细胞毒性 T 淋巴细胞 (CTL)。焦亡引起的免疫反应可以将免疫抑制性“冷”肿瘤微环境(TME)转变为免疫原性“热”TME,这不仅抑制原发性胰腺癌的生长,而且攻击远处的肿瘤。该工作为细胞焦亡引起的光控抗肿瘤免疫和实体瘤免疫治疗建立了一个具有高生物相容性的平台。

































 京公网安备 11010802027423号
京公网安备 11010802027423号