当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Bioevaluation of a Novel Hybrid Molecular Pyrrolobenzodiazepine–Anthracenecarboxyimide as a Payload for Antibody–Drug Conjugate
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-08-18 , DOI: 10.1021/acs.jmedchem.2c00471 Weirong Lai 1 , Shengyan Zhao 1 , Qinhuai Lai 1 , Wei Zhou 1 , Mengdan Wu 1 , Xiaohua Jiang 1 , Xin Wang 1 , Yujia Peng 1 , Xian Wei 1 , Liang Ouyang 1 , Lantu Gou 1 , Hao Chen 2 , Yuxi Wang 1, 3 , Jinliang Yang 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-08-18 , DOI: 10.1021/acs.jmedchem.2c00471 Weirong Lai 1 , Shengyan Zhao 1 , Qinhuai Lai 1 , Wei Zhou 1 , Mengdan Wu 1 , Xiaohua Jiang 1 , Xin Wang 1 , Yujia Peng 1 , Xian Wei 1 , Liang Ouyang 1 , Lantu Gou 1 , Hao Chen 2 , Yuxi Wang 1, 3 , Jinliang Yang 1
Affiliation
|
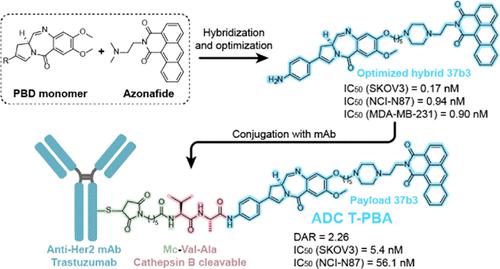
A novel series of hybrid molecules combining pyrrolobenzodiazepine (PBD) and anthracenecarboxyimide pharmacophores were designed, synthesized, and tested for in vitro cytotoxicity against various cancer cell lines. The most potent compound from this series, 37b3, exhibited a subnanomolar level of cytotoxicity with an IC50 of 0.17–0.94 nM. 37b3 induced DNA damage and led to tumor cell cycle arrest and apoptosis. We employed 37b3 as a payload to conjugate with trastuzumab to obtain the antibody–drug conjugate (ADC) T-PBA. T-PBA maintained its mode of target and internalization ability of trastuzumab. We demonstrated that T-PBA could be degraded through the lysosomal pathway to release the payload 37b3 after internalization. T-PBA showed a powerful killing effect on Her2-positive cancer cells in vitro. Furthermore, T-PBA significantly inhibited tumor growth in gastric and ovarian cancer xenograft mouse models without overt toxicity. Collectively, these studies suggest that T-PBA represents a promising new ADC that deserves further investigation.
中文翻译:

新型杂化分子吡咯并苯二氮卓-蒽羧酰亚胺作为抗体-药物偶联物有效载荷的设计、合成和生物评价
设计、合成了一系列结合吡咯并苯二氮卓 (PBD) 和蒽羧酰亚胺药效团的新型混合分子,并测试了其对各种癌细胞系的体外细胞毒性。该系列中最有效的化合物37b3表现出亚纳摩尔水平的细胞毒性,IC 50为 0.17–0.94 nM。37b3诱导 DNA 损伤并导致肿瘤细胞周期停滞和细胞凋亡。我们使用37b3作为有效载荷与曲妥珠单抗偶联以获得抗体-药物偶联物 (ADC) T-PBA。T-PBA保持了曲妥珠单抗的靶向模式和内化能力。我们证明了 T-PBA 可以通过溶酶体途径降解以释放有效载荷37b3内化后。T-PBA在体外对 Her2 阳性癌细胞显示出强大的杀伤作用。此外,T-PBA 显着抑制胃癌和卵巢癌异种移植小鼠模型中的肿瘤生长,而没有明显的毒性。总的来说,这些研究表明 T-PBA 代表了一种有前途的新 ADC,值得进一步研究。
更新日期:2022-08-18
中文翻译:

新型杂化分子吡咯并苯二氮卓-蒽羧酰亚胺作为抗体-药物偶联物有效载荷的设计、合成和生物评价
设计、合成了一系列结合吡咯并苯二氮卓 (PBD) 和蒽羧酰亚胺药效团的新型混合分子,并测试了其对各种癌细胞系的体外细胞毒性。该系列中最有效的化合物37b3表现出亚纳摩尔水平的细胞毒性,IC 50为 0.17–0.94 nM。37b3诱导 DNA 损伤并导致肿瘤细胞周期停滞和细胞凋亡。我们使用37b3作为有效载荷与曲妥珠单抗偶联以获得抗体-药物偶联物 (ADC) T-PBA。T-PBA保持了曲妥珠单抗的靶向模式和内化能力。我们证明了 T-PBA 可以通过溶酶体途径降解以释放有效载荷37b3内化后。T-PBA在体外对 Her2 阳性癌细胞显示出强大的杀伤作用。此外,T-PBA 显着抑制胃癌和卵巢癌异种移植小鼠模型中的肿瘤生长,而没有明显的毒性。总的来说,这些研究表明 T-PBA 代表了一种有前途的新 ADC,值得进一步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号