Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2022-08-18 , DOI: 10.1016/j.jphotochem.2022.114217 Róbert Balogh , Anita Eckstein , Kamil Tokár , Martin Danko
|
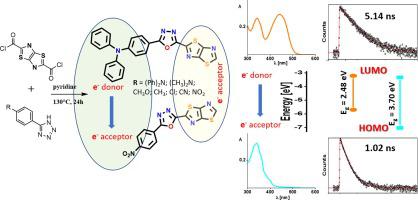
The present research explores the experimental and theoretical study of seven new asymmetric derivatives with thiazolo[5,4-d]thiazole acceptor block and para substituted phenyl functional donor and acceptor groups linked with oxadiazole unit. We present one pot preparation of small organic molecules by simple condensation of commercially available tetrazoles and easily synthesized acyl chloride of thiazolo[5,4-d]thiazole unit. For a deeper examination of the products, UV-Vis, steady-state, and time-correlated fluorescence spectroscopy in chloroform solutions were used to study the correlation between the structure and the spectroscopic behavior of prepared compounds. The electronic properties of these derivatives were experimentally studied by cyclic voltammetry. The molecules were further investigated by quantum chemical calculations. It is almost certain, that studied compounds with weak electron-donor and medium to strong electron acceptor ability are not low band gap electron transport materials because UV–Vis and cyclic voltammetry results supported by the theoretical study show a wide band gap between their HOMO and LUMO orbitals. However, they may be useful in wide-bandgap semiconductors. Derivatives with electron donor moieties show low band gap and intramolecular charge transfer in the visible light region which makes them promising candidates for organic electronics. This study lays the groundwork for future research of thiazolo[5,4-d] thiazole molecules with 1,3,4-oxadiazole linkers.
中文翻译:

噻唑并[5,4-d]噻唑基小分子的合成及光谱研究,以1,3,4-恶二唑为有机电子连接剂
本研究探索了七种新型不对称衍生物的实验和理论研究,其中噻唑并[5,4- d ]噻唑受体嵌段和对位取代的苯基功能性供体和受体基团与恶二唑单元相连。我们提出了通过简单缩合市售四唑和易于合成的噻唑酰氯的一锅法制备有机小分子[5,4- d]噻唑单元。为了更深入地检查产品,使用氯仿溶液中的紫外-可见、稳态和时间相关荧光光谱来研究所制备化合物的结构和光谱行为之间的相关性。通过循环伏安法对这些衍生物的电子性质进行了实验研究。通过量子化学计算进一步研究了这些分子。几乎可以肯定的是,所研究的具有弱电子供体和中等至强电子受体能力的化合物不是低带隙电子传输材料,因为理论研究支持的紫外-可见光和循环伏安法结果表明它们的 HOMO 和LUMO 轨道。然而,它们可能在宽带隙半导体中有用。具有电子供体部分的衍生物在可见光区域显示出低带隙和分子内电荷转移,这使其成为有机电子学的有希望的候选者。本研究为噻唑啉的未来研究奠定了基础[5,4-d ] 具有 1,3,4-恶二唑接头的噻唑分子。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号