International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2022-08-18 , DOI: 10.1016/j.ijhydene.2022.07.026
Qiongqiong Wang , Yaxi Tian , Mengyun Chen , Rongfeng Guan , Haibin Yuan
|
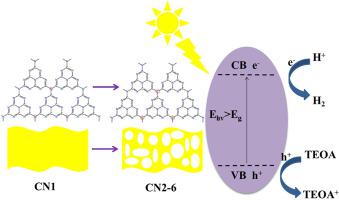
Photocatalytic production H2 of splitting water is an important branch of the application of photocatalytic materials. In this paper, porous C doped carbon nitride was successfully synthesized by calcining urea and acetamide at high temperature, and then analyzed by various characterization methods. XRD and FT-IR spectra show that the crystal structure of carbon nitride modified by acetamide are similar to bulk carbon nitride. SEM and TEM spectra show that the morphology and structure of carbon nitride and carbon nitride was modified by acetamide, which presents a porous nanosheets structure. XPS and EDS show that the content of carbon element in carbon nitride modified by acetamide increased significantly. The PL spectrum is obviously red shift, and the light absorption range is obviously enlarged, which is consistent with the absorption edge of UV–vis Spectrum. The BET of the optimized sample CN2-6 is 122.79 m2g-1, which is 3.04 times higher than that of bulk carbon nitride CN1 (40.456 m2g-1). In addition, the hydrogen production of CN2-6 is 13169.04 μmol/g/h under 300 W Xenon lamp, which is 14.95 times more than CN1 (880.82 μmol/g/h) hydrogen production rate. When the light source is a 10 W LED lamp, the apparent quantum efficiency (AQE) of sample CN2-6 reaches the maximum (2.91) under the wavelengths of 430 nm, which is 1.27 times more than CN1 under the same conditions. Meanwhile, prepared samples have excellent stability and durability under visible light.
中文翻译:

乙酰胺控制的多孔C掺杂g-C3N4纳米片的制备用于光催化析氢
光催化生产H 2水分解是光催化材料应用的一个重要分支。本文通过高温煅烧尿素和乙酰胺,成功合成了多孔C掺杂氮化碳,并通过多种表征方法进行了分析。XRD和FT-IR光谱表明乙酰胺改性氮化碳的晶体结构与块状氮化碳相似。SEM和TEM光谱表明,氮化碳和氮化碳的形貌和结构被乙酰胺修饰,呈现出多孔纳米片结构。XPS和EDS表明乙酰胺改性氮化碳中碳元素含量显着增加。PL光谱明显红移,光吸收范围明显扩大,与UV-vis光谱的吸收边一致。2 g -1 ,是块状氮化碳CN1(40.456 m 2 g -1 )的3.04倍。此外,CN2-6在300 W氙灯下的产氢量为13169.04 μmol/g/h,是CN1(880.82 μmol/g/h)产氢率的14.95倍。当光源为10 W LED灯时,样品CN2-6的表观量子效率(AQE)在430 nm波长下达到最大值(2.91),是相同条件下CN1的1.27倍。同时,制备的样品在可见光下具有优异的稳定性和耐久性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号