当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On-Demand Photochemical Synthesis of Hydrogen Peroxide from Alkylated Anthraquinones
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-08-18 , DOI: 10.1021/acssuschemeng.2c01404 Hunter B. Vibbert 1, 2 , Christoph Bendel 3 , Jack R. Norton 3 , Aaron J. Moment 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-08-18 , DOI: 10.1021/acssuschemeng.2c01404 Hunter B. Vibbert 1, 2 , Christoph Bendel 3 , Jack R. Norton 3 , Aaron J. Moment 2
Affiliation
|
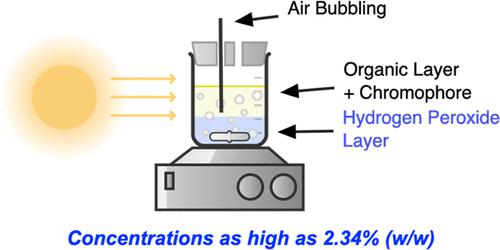
Direct illumination of anthraquinones with light, in air, to produce H2O2 reliably and robustly has been developed for aqueous, electrolyte-free, highly pure H2O2 production. Effects of chromophore, solvent, air saturation, and light source have been investigated to understand the mechanism of this transformation. Upon photosensitization of an air-saturated, biphasic solution consisting of an organic solvent and water, H2O2 can be quickly and linearly produced with the oxidized solvent remaining in the organic phase. The mechanism of this transformation has been probed using computational methods, suggesting that proton-coupled electron transfer from an organic solvent to an anthraquinone catalyst is the most likely mechanistic pathway. Commercially relevant H2O2 concentrations, as high as 2.34% (w/w), have been produced in 3 h, among the fastest photochemical H2O2 production reaction reported.
中文翻译:

烷基化蒽醌按需光化学合成过氧化氢
已开发出在空气中用光直接照射蒽醌,以可靠且稳健地生产 H 2 O 2 ,用于水性、无电解质、高纯度 H 2 O 2生产。已经研究了发色团、溶剂、空气饱和度和光源的影响,以了解这种转变的机制。在由有机溶剂和水组成的空气饱和双相溶液的光敏作用下,H 2 O 2可以在有机相中残留的氧化溶剂快速线性生产。已经使用计算方法探索了这种转变的机制,表明从有机溶剂到蒽醌催化剂的质子耦合电子转移是最可能的机制途径。商业相关的 H 2 O 2浓度高达 2.34% (w/w),已在 3 小时内产生,是报道的最快的光化学 H 2 O 2生产反应之一。
更新日期:2022-08-18
中文翻译:

烷基化蒽醌按需光化学合成过氧化氢
已开发出在空气中用光直接照射蒽醌,以可靠且稳健地生产 H 2 O 2 ,用于水性、无电解质、高纯度 H 2 O 2生产。已经研究了发色团、溶剂、空气饱和度和光源的影响,以了解这种转变的机制。在由有机溶剂和水组成的空气饱和双相溶液的光敏作用下,H 2 O 2可以在有机相中残留的氧化溶剂快速线性生产。已经使用计算方法探索了这种转变的机制,表明从有机溶剂到蒽醌催化剂的质子耦合电子转移是最可能的机制途径。商业相关的 H 2 O 2浓度高达 2.34% (w/w),已在 3 小时内产生,是报道的最快的光化学 H 2 O 2生产反应之一。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号