当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific Nitrogen Insertion Using Amino Diphenylphosphinates: An Aza-Baeyer–Villiger Rearrangement
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-17 , DOI: 10.1021/acs.orglett.2c02361 Mike Ong 1, 2 , Marlene Arnold 2 , Alexander W Walz 2 , Johannes M Wahl 2
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-17 , DOI: 10.1021/acs.orglett.2c02361 Mike Ong 1, 2 , Marlene Arnold 2 , Alexander W Walz 2 , Johannes M Wahl 2
Affiliation
|
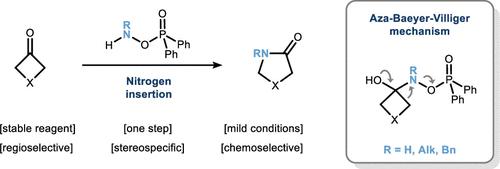
Amino diphenylphosphinates, which are commercially available or easily prepared from hydroxylamine, undergo ring expansion of cyclobutanones toward γ-lactams under mild conditions. A reaction pathway profoundly different from the common Beckmann reaction is achieved through the ambivalent character of the aminating agent. Thus, rearrangement occurs from a Criegee-like intermediate prior to the formation of the oxime species, which is corroborated by mechanistic experiments. Based on this observation, the migrating aptitude of the adjacent groups is analyzed and found to be in line with the parent Baeyer–Villiger reaction rendering a regioselective (up to >99:1 rr), stereospecific (>99% enantiospecificity), and chemoselective (>99%) insertion process possible. The method thus qualifies for late-stage skeletal editing as showcased by the synthesis of Rolipram and its N-alkylated analogs.
中文翻译:

使用氨基二苯基次膦酸盐的立体特异性氮插入:Aza-Baeyer-Villiger 重排
可商购或容易从羟胺制备的氨基二苯基次膦酸盐在温和条件下经受环丁酮向γ-内酰胺的扩环。通过胺化剂的矛盾特征实现了与常见贝克曼反应截然不同的反应途径。因此,重排从类似 Criegee 的中间体发生在肟物种形成之前,机械实验证实了这一点。基于这一观察,分析了相邻组的迁移能力,发现其与母体 Baeyer-Villiger 反应一致,呈现区域选择性(高达 >99:1 rr)、立体特异性(>99% 对映体特异性)和化学选择性(>99%) 插入过程可能。N-烷基化类似物。
更新日期:2022-08-17
中文翻译:

使用氨基二苯基次膦酸盐的立体特异性氮插入:Aza-Baeyer-Villiger 重排
可商购或容易从羟胺制备的氨基二苯基次膦酸盐在温和条件下经受环丁酮向γ-内酰胺的扩环。通过胺化剂的矛盾特征实现了与常见贝克曼反应截然不同的反应途径。因此,重排从类似 Criegee 的中间体发生在肟物种形成之前,机械实验证实了这一点。基于这一观察,分析了相邻组的迁移能力,发现其与母体 Baeyer-Villiger 反应一致,呈现区域选择性(高达 >99:1 rr)、立体特异性(>99% 对映体特异性)和化学选择性(>99%) 插入过程可能。N-烷基化类似物。































 京公网安备 11010802027423号
京公网安备 11010802027423号