Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Wireframe Orbit-Accelerated Bipedal DNA Walker for Electrochemiluminescence Detection of Methyltransferase Activity
ACS Sensors ( IF 8.2 ) Pub Date : 2022-08-17 , DOI: 10.1021/acssensors.2c01262 Mei-Chen Pan 1 , Mao-Hua Gao 1 , Xia Yang 1 , Wen-Bin Liang 1 , Ruo Yuan 1 , Ying Zhuo 1
ACS Sensors ( IF 8.2 ) Pub Date : 2022-08-17 , DOI: 10.1021/acssensors.2c01262 Mei-Chen Pan 1 , Mao-Hua Gao 1 , Xia Yang 1 , Wen-Bin Liang 1 , Ruo Yuan 1 , Ying Zhuo 1
Affiliation
|
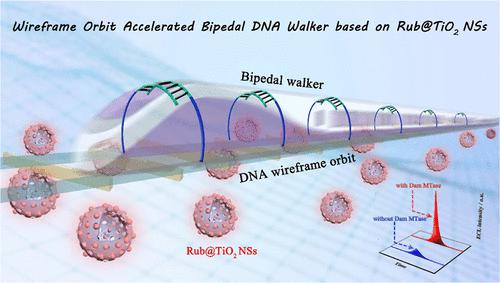
In spite of the DNA walkers executing the signal accumulation task in the process of moving along the predetermined paths, the enhancement of walking dynamics and walking path controllability are still challenging due to the unprogrammed arrangements of DNA orbits. Taking these dilemmas into account, a bipedal DNA walker was designed skillfully by the virtue of wireframe orbits assembled by DNA cubes in order, which improved the efficiency and the continuity of walking. It could be attributed to the fact that both the contact chance and the dynamic interaction between walking strands and designated orbits were beneficial to minimize the possibility of derailment and improve the accumulation of signal. In addition, the hollow titanium dioxide nanospheres coated with rubrene (Rub@TiO2 NSs) were prepared by the etching of inner silicon dioxide nanoparticles (SiO2 NPs) to regulate the distribution pattern of rubrene (Rub) molecules and expose more electrochemically active sites for high-efficient electrochemiluminescence (ECL). Benefiting by the pore confinement-enhanced ECL, the electron and mass transfer was significantly accelerated because of the hollow structure of Rub@TiO2 NSs. Subsequently, endogenous dissolved oxygen as the coreactant and palladium nanoparticles (Pd NPs) as the coreaction accelerator were employed to constitute a ternary ECL system with explosive signal response. Combining with this ECL platform, the bipedal walker activated by the target can autonomously and directionally move on the DNA wireframe orbits to release the quenching probes continuously. In this way, the biosensor displayed a low detection limit (2.30 × 10–8 U·mL–1) and a wide linear range (1.0 × 10–7 to 1.0 × 10–1 U·mL–1) for the sensitive detection of Dam methyltransferase (Dam MTase) activity. Therefore, a novel strategy for the accurate quantification of epigenetic targets was developed by virtue of improving the walking dynamics of DNA walker and amplifying the ECL of Rub molecules.
中文翻译:

用于甲基转移酶活性电化学发光检测的线框轨道加速双足 DNA Walker
尽管 DNA 步行者在沿着预定路径移动的过程中执行信号积累任务,但由于 DNA 轨道的未编程安排,步行动力学和步行路径可控性的增强仍然具有挑战性。考虑到这些困境,利用DNA立方体有序组装的线框轨道,巧妙地设计了双足DNA步行器,提高了步行效率和连续性。这可以归因于这样一个事实,即步行链与指定轨道之间的接触机会和动态相互作用有利于最大限度地减少脱轨的可能性并改善信号的积累。此外,包覆红荧烯的空心二氧化钛纳米球(Rub@TiO 2NSs) 通过蚀刻内部二氧化硅纳米粒子 (SiO 2 NPs) 制备,以调节红荧烯 (Rub) 分子的分布模式并暴露更多电化学活性位点以实现高效电化学发光 (ECL)。受益于孔限制增强的ECL,由于Rub@TiO 2的中空结构,电子和质量转移显着加速NS。随后,采用内源溶解氧作为共反应物和钯纳米粒子(Pd NPs)作为共反应促进剂,构成了具有爆炸信号响应的三元ECL体系。结合这个ECL平台,被目标激活的双足步行器可以在DNA线框轨道上自主定向移动,持续释放淬灭探针。通过这种方式,生物传感器显示出低检测限 (2.30 × 10 –8 U·mL –1 ) 和宽线性范围 (1.0 × 10 –7至 1.0 × 10 –1 U·mL –1 )) 用于灵敏检测 Dam 甲基转移酶 (Dam MTase) 活性。因此,通过改善 DNA 步行者的行走动力学和放大 Rub 分子的 ECL,开发了一种准确量化表观遗传目标的新策略。
更新日期:2022-08-17
中文翻译:

用于甲基转移酶活性电化学发光检测的线框轨道加速双足 DNA Walker
尽管 DNA 步行者在沿着预定路径移动的过程中执行信号积累任务,但由于 DNA 轨道的未编程安排,步行动力学和步行路径可控性的增强仍然具有挑战性。考虑到这些困境,利用DNA立方体有序组装的线框轨道,巧妙地设计了双足DNA步行器,提高了步行效率和连续性。这可以归因于这样一个事实,即步行链与指定轨道之间的接触机会和动态相互作用有利于最大限度地减少脱轨的可能性并改善信号的积累。此外,包覆红荧烯的空心二氧化钛纳米球(Rub@TiO 2NSs) 通过蚀刻内部二氧化硅纳米粒子 (SiO 2 NPs) 制备,以调节红荧烯 (Rub) 分子的分布模式并暴露更多电化学活性位点以实现高效电化学发光 (ECL)。受益于孔限制增强的ECL,由于Rub@TiO 2的中空结构,电子和质量转移显着加速NS。随后,采用内源溶解氧作为共反应物和钯纳米粒子(Pd NPs)作为共反应促进剂,构成了具有爆炸信号响应的三元ECL体系。结合这个ECL平台,被目标激活的双足步行器可以在DNA线框轨道上自主定向移动,持续释放淬灭探针。通过这种方式,生物传感器显示出低检测限 (2.30 × 10 –8 U·mL –1 ) 和宽线性范围 (1.0 × 10 –7至 1.0 × 10 –1 U·mL –1 )) 用于灵敏检测 Dam 甲基转移酶 (Dam MTase) 活性。因此,通过改善 DNA 步行者的行走动力学和放大 Rub 分子的 ECL,开发了一种准确量化表观遗传目标的新策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号