Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2022-08-17 , DOI: 10.1016/j.molliq.2022.120095 Yuanyuan Yue , Qimin Tu , Yiying Guo , Yunting Wang , Yue Xu , Yilin Zhang , Jianming Liu
|
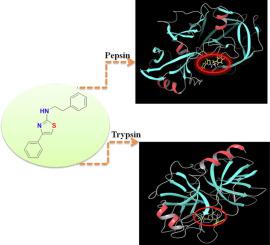
In this work, the binding affinity of fanetizole with proteases pepsin and trypsin were investigated by spectroscopy and molecular docking. As a thiazole derivative, the interactions of fanetizole with either pepsin or trypsin resulted in a fluorescence quenching. As such, the calculated values of K manifested that the fanetizole-pepsin system has a stronger binding affinity than fanetizole-trypsin at 296 K. Moreover, steady-state, time-resolved fluorescence and thermodynamic studies of the interaction of fanetizole with pepsin or trypsin suggested that a static fluorescence quenching mechanism and the interactions were primarily attributed to hydrogen-bonding interaction. The observed changes in synchronous fluorescence, circular dichroism spectroscopy, and FTIR results were related to the secondary construction alternations of both proteases. In line with fluorescence spectroscopy, molecular docking demonstrated similar binding characteristics and domains. The range of r values showed energy transfer from pepsin/trypsin to fanetizole.
中文翻译:

法奈唑唑与胃蛋白酶和胰蛋白酶相互作用的比较:光谱和分子对接方法
在这项工作中,通过光谱学和分子对接研究了fanetizole与蛋白酶胃蛋白酶和胰蛋白酶的结合亲和力。作为噻唑衍生物,fanetizole 与胃蛋白酶或胰蛋白酶的相互作用导致荧光猝灭。因此,K 的计算值表明,在 296 K 时,fanetizole-pepsin 系统具有比 fanetizole-trypsin 更强的结合亲和力。此外,fanetizole 与胃蛋白酶或胰蛋白酶相互作用的稳态、时间分辨荧光和热力学研究表明静态荧光猝灭机制和相互作用主要归因于氢键相互作用。观察到的同步荧光、圆二色光谱和 FTIR 结果的变化与两种蛋白酶的二级结构交替有关。与荧光光谱一致,分子对接表现出相似的结合特征和结构域。r 值范围显示从胃蛋白酶/胰蛋白酶到法奈唑的能量转移。












































 京公网安备 11010802027423号
京公网安备 11010802027423号