当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Continuous-Flow Synthesis of syn-2-Amino-1,3-diol via Catalytic Hydrogenation: A Vital Intermediate of (+)-Thiamphenicol and (+)-Florfenicol
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-08-17 , DOI: 10.1021/acs.oprd.2c00100 Yingqi Xia 1 , Baijun Ye 2 , Minjie Liu 2 , Meifen Jiang 2 , Fener Chen 1, 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-08-17 , DOI: 10.1021/acs.oprd.2c00100 Yingqi Xia 1 , Baijun Ye 2 , Minjie Liu 2 , Meifen Jiang 2 , Fener Chen 1, 2
Affiliation
|
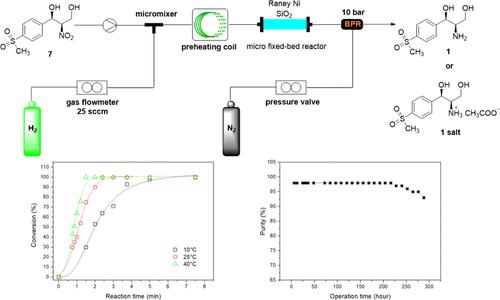
In this paper, an expeditious and efficient continuous-flow process is reported for the synthesis of syn-2-amino-1,3-diol. The starting material syn-2-nitro-1,3-diol reacts with hydrogen gas in a micro fixed-bed reactor packed with a Raney Ni catalyst to provide syn-2-amino-1,3-diol in 93% isolated yield. The reaction time in the flow system was markedly reduced (from 36 h in batch to 5 min in flow) due to enhanced mass transfer. The effects of the catalyst, solvent, reaction temperature, reaction time, pressure, and H2 flow rate have been investigated successively. The optimum conditions for the continuous-flow reaction are described as follows when using Raney Ni as a catalyst at a reaction temperature of 25 °C, a reaction pressure of 10 bar, a reaction solvent of 3% AcOH in methanol, an H2 flow rate of 25 sccm, and a liquid flow rate of 0.6 mL/min. The reaction kinetics in the temperature range of 10–40 °C were investigated. The reaction rate constants and activation energy were determined. The continuous flow system allows for an efficient hydrogenation of syn-2-nitro-1,3-diol on the hectogram scale, corresponding to an overall productivity of 73.6 g/d after continuous operation for over 240 h.
中文翻译:

通过催化加氢连续流合成 syn-2-Amino-1,3-二醇:(+)-甲砜霉素和 (+)-氟苯尼考的重要中间体
本文报道了一种快速有效的连续流动工艺合成顺-2-氨基-1,3-二醇。起始原料syn -2-nitro-1,3-diol 在装有阮内镍催化剂的微型固定床反应器中与氢气反应,以 93% 的分离产率提供syn -2-amino-1,3-diol。由于增强的传质,流动系统中的反应时间显着减少(从分批 36 小时到流动 5 分钟)。催化剂、溶剂、反应温度、反应时间、压力和 H 2的影响流量已被陆续研究。以阮内镍为催化剂,反应温度为 25 ℃,反应压力为 10 bar,反应溶剂为 3% AcOH 的甲醇溶液,H 2流量时,连续流动反应的最佳条件如下:流速为 25 sccm,液体流速为 0.6 mL/min。研究了10-40°C温度范围内的反应动力学。测定反应速率常数和活化能。连续流动系统允许在百克规模上对顺-2-硝基-1,3-二醇进行有效加氢,在连续运行超过 240 小时后,总产率为 73.6 g/d。
更新日期:2022-08-17
中文翻译:

通过催化加氢连续流合成 syn-2-Amino-1,3-二醇:(+)-甲砜霉素和 (+)-氟苯尼考的重要中间体
本文报道了一种快速有效的连续流动工艺合成顺-2-氨基-1,3-二醇。起始原料syn -2-nitro-1,3-diol 在装有阮内镍催化剂的微型固定床反应器中与氢气反应,以 93% 的分离产率提供syn -2-amino-1,3-diol。由于增强的传质,流动系统中的反应时间显着减少(从分批 36 小时到流动 5 分钟)。催化剂、溶剂、反应温度、反应时间、压力和 H 2的影响流量已被陆续研究。以阮内镍为催化剂,反应温度为 25 ℃,反应压力为 10 bar,反应溶剂为 3% AcOH 的甲醇溶液,H 2流量时,连续流动反应的最佳条件如下:流速为 25 sccm,液体流速为 0.6 mL/min。研究了10-40°C温度范围内的反应动力学。测定反应速率常数和活化能。连续流动系统允许在百克规模上对顺-2-硝基-1,3-二醇进行有效加氢,在连续运行超过 240 小时后,总产率为 73.6 g/d。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号