Food Hydrocolloids ( IF 11.0 ) Pub Date : 2022-08-13 , DOI: 10.1016/j.foodhyd.2022.108008 Hongling Fu , Jiaxin Li , Xiaoqing Yang , Mohammed Sharif Swallah , Hao Gong , Lei Ji , Xiangze Meng , Bo Lyu , Hansong Yu
|
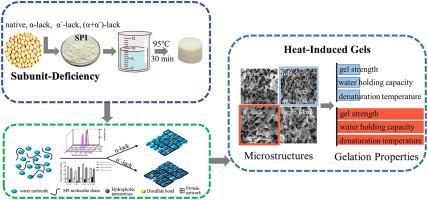
Soy protein isolate (SPI) is widely used in the food industry as a gelling agent due to its high water-holding capacity and gelation. As a key in elucidating the structure-function relationships of SPI, subunit composition can explain many functional properties of soy protein. In this study, to explore the influence of α and α′ subunits on the gel properties of soy protein, the gel strength and water holding capacity of the heat-induced gel with missing subunits were determined. Texture Profile Analysis (TPA) showed that gel strength from large to small was α′-lack > (α and α′)-lack > native > α-lack, and the lack of α′ subunit significantly enhanced (p < 0.05) the gel strength and water-holding capacity of the resultant gels. In the gel formation process, microstructure observation of the gels revealed that the α′-lacking gel had a more uniform and denser network structure with higher storage modulus, promoting more water molecules to be bound in the gel structure as immobilized water and exhibiting higher thermal stability. Subsequently, the lack of α′ subunit increased the zeta potential, surface hydrophobicity (H0), and fluorescence intensity of the gel. The deletion of the α′ subunit promoted the exposure of hydrophobic groups with more hydrophobic amino acids and promoted the aggregation of the subunits after heat treatment. These changes in the physicochemical properties of the gel affected their heat-induced gelation. In addition, gel solubility results signify that hydrophobic and disulfide bond interactions are the main forces in the gel formation process. Thus, the deletion of the α′ subunit will facilitate the development and application of soy protein products in food processing and improve their gel properties. This study will benefit the application of soy protein at the subunit level in traditional dairy products based on heated-induced gelation.
中文翻译:

亚基水平大豆分离蛋白的加热诱导凝胶化:基于天然杂交育种探索α和α'亚基对SPI凝胶化的影响
大豆分离蛋白 (SPI) 由于其高保水性和凝胶化特性,在食品工业中被广泛用作胶凝剂。作为阐明 SPI 结构-功能关系的关键,亚基组成可以解释大豆蛋白的许多功能特性。本研究为探讨α和α'亚基对大豆蛋白凝胶性质的影响,测定了缺失亚基的热诱导凝胶的凝胶强度和持水能力。Texture Profile Analysis (TPA) 表明凝胶强度由大到小为 α′-lack > (α and α′)-lack > native > α-lack, α′亚基缺失明显增强( p < 0.05) 所得凝胶的凝胶强度和持水能力。在凝胶形成过程中,凝胶的微观结构观察表明,缺乏α'的凝胶具有更均匀和更致密的网络结构,具有更高的储能模量,促进更多的水分子作为固定水结合在凝胶结构中,表现出更高的热稳定。随后,缺乏 α' 亚基增加了 zeta 电位、表面疏水性(H 0) 和凝胶的荧光强度。α'亚基的缺失促进了具有更多疏水氨基酸的疏水基团的暴露,并促进了热处理后亚基的聚集。凝胶物理化学性质的这些变化影响了它们的热诱导凝胶化。此外,凝胶溶解度结果表明疏水和二硫键相互作用是凝胶形成过程中的主要力量。因此,α'亚基的缺失将促进大豆蛋白产品在食品加工中的开发和应用,并改善其凝胶性能。本研究将有利于大豆蛋白在亚基水平上在基于加热诱导凝胶化的传统乳制品中的应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号