Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of 3,4-dihydropyrrolo[1,2-a]pyrazin-1(2H)-one derivatives as PIM kinase inhibitors inspired from marine alkaloids
Chirality ( IF 2.8 ) Pub Date : 2022-08-12 , DOI: 10.1002/chir.23501 Francesco Casuscelli 1, 2 , Elena Ardini 1 , Nilla Avanzi 1 , Alessandra Badari 1 , Elena Casale 1 , Teresa Disingrini 1 , Daniele Donati 1 , Antonella Ermoli 1 , Eduard R Felder 1 , Arturo Galvani 1 , Antonella Isacchi 1 , Maria Menichincheri 1 , Marisa Montemartini 1 , Christian Orrenius 1 , Claudia Piutti 2 , Barbara Salom 1 , Gianluca Papeo 1
Chirality ( IF 2.8 ) Pub Date : 2022-08-12 , DOI: 10.1002/chir.23501 Francesco Casuscelli 1, 2 , Elena Ardini 1 , Nilla Avanzi 1 , Alessandra Badari 1 , Elena Casale 1 , Teresa Disingrini 1 , Daniele Donati 1 , Antonella Ermoli 1 , Eduard R Felder 1 , Arturo Galvani 1 , Antonella Isacchi 1 , Maria Menichincheri 1 , Marisa Montemartini 1 , Christian Orrenius 1 , Claudia Piutti 2 , Barbara Salom 1 , Gianluca Papeo 1
Affiliation
|
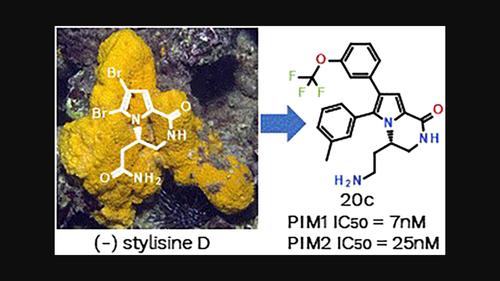
We previously demonstrated that natural product-inspired 3,4-dihydropyrrolo[1,2-a]pyrazin-1(2H)-ones derivatives delivered potent and selective PIM kinases inhibitors however with non-optimal ADME/PK properties and modest oral bioavailability. Herein, we describe a structure-based scaffold decoration and a stereoselective approach to this chemical class. The synthesis, structure–activity relationship studies, chiral analysis, and pharmacokinetic data of compounds from this inhibitor class are presented herein. Compound 20c demonstrated excellent potency on PIM1 and PIM2 with exquisite kinases selectivity and PK properties that efficiently and dose-dependently promoted c-Myc degradation and appear to be promising lead compounds for further development.
中文翻译:

受海洋生物碱启发,立体选择性合成 3,4-二氢吡咯并[1,2-a]吡嗪-1(2H)-one 衍生物作为 PIM 激酶抑制剂
我们之前证明了受天然产物启发的 3,4-二氢吡咯并[1,2-a]吡嗪-1(2H)-ones 衍生物可提供有效和选择性的 PIM 激酶抑制剂,但具有非最佳的 ADME/PK 特性和适度的口服生物利用度。在这里,我们描述了一种基于结构的支架装饰和这种化学类别的立体选择性方法。本文介绍了此类抑制剂化合物的合成、构效关系研究、手性分析和药代动力学数据。化合物20c对 PIM1 和 PIM2 表现出优异的效力,具有出色的激酶选择性和 PK 特性,可有效且剂量依赖性地促进 c-Myc 降解,并且似乎是有希望进一步开发的先导化合物。
更新日期:2022-08-12
中文翻译:

受海洋生物碱启发,立体选择性合成 3,4-二氢吡咯并[1,2-a]吡嗪-1(2H)-one 衍生物作为 PIM 激酶抑制剂
我们之前证明了受天然产物启发的 3,4-二氢吡咯并[1,2-a]吡嗪-1(2H)-ones 衍生物可提供有效和选择性的 PIM 激酶抑制剂,但具有非最佳的 ADME/PK 特性和适度的口服生物利用度。在这里,我们描述了一种基于结构的支架装饰和这种化学类别的立体选择性方法。本文介绍了此类抑制剂化合物的合成、构效关系研究、手性分析和药代动力学数据。化合物20c对 PIM1 和 PIM2 表现出优异的效力,具有出色的激酶选择性和 PK 特性,可有效且剂量依赖性地促进 c-Myc 降解,并且似乎是有希望进一步开发的先导化合物。













































 京公网安备 11010802027423号
京公网安备 11010802027423号