Cell Reports ( IF 7.5 ) Pub Date : 2022-08-09 , DOI: 10.1016/j.celrep.2022.111175
Josephine Bock 1 , Nathalie Kühnle 2 , Julia D Knopf 1 , Nina Landscheidt 2 , Jin-Gu Lee 3 , Yihong Ye 3 , Marius K Lemberg 1
|
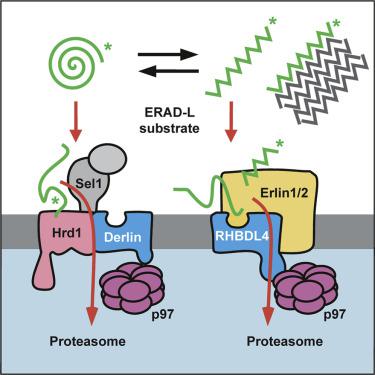
Protein degradation is fundamentally important to ensure cell homeostasis. In the endoplasmic reticulum (ER), the ER-associated degradation (ERAD) pathway targets incorrectly folded and unassembled proteins for turnover by the cytoplasmic proteasome. Previously, we showed that the rhomboid protease RHBDL4, together with p97, mediates membrane protein degradation. However, whether RHBDL4 acts in concert with additional ERAD components is unclear, and its full substrate spectrum remains to be defined. Here, we show that, in addition to membrane proteins, RHBDL4 cleaves aggregation-prone luminal ERAD substrates. Since mutations of the RHBDL4 rhomboid domain led to stabilization of substrates at the cytoplasmic side, we hypothesize that, analogous to the homolog ERAD factor derlin, RHBDL4 is directly involved in substrate retrotranslocation. RHBDL4's interaction with the erlin ERAD complex and reciprocal interaction of rhomboid substrates with erlins suggest that RHBDL4 and erlins form a complex that clips substrates and thereby rescues aggregation-prone peptides in the ER from aggregation.
中文翻译:

菱形蛋白酶 RHBDL4 促进易聚集蛋白的逆向易位以进行降解
蛋白质降解对于确保细胞稳态至关重要。在内质网 (ER) 中,ER 相关降解 (ERAD) 途径靶向错误折叠和未组装的蛋白质,以通过细胞质蛋白酶体进行转换。以前,我们发现菱形蛋白酶 RHBDL4 与 p97 一起介导膜蛋白降解。然而,RHBDL4 是否与其他 ERAD 成分协同作用尚不清楚,其完整的底物谱仍有待确定。在这里,我们表明,除了膜蛋白外,RHBDL4 还切割易于聚集的腔 ERAD 底物。由于 RHBDL4 菱形结构域的突变导致底物在细胞质侧稳定,我们假设,类似于同源 ERAD 因子 derlin,RHBDL4 直接参与底物逆行易位。

































 京公网安备 11010802027423号
京公网安备 11010802027423号