当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C–H acylation of aniline derivatives with α-oxocarboxylic acids using ruthenium catalyst
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2ob01212j Qiong Liu 1 , Jia-Yuan Yong 1 , Jing Zhang 2 , Tao Ban 1 , Xu-Qin Li 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2ob01212j Qiong Liu 1 , Jia-Yuan Yong 1 , Jing Zhang 2 , Tao Ban 1 , Xu-Qin Li 1
Affiliation
|
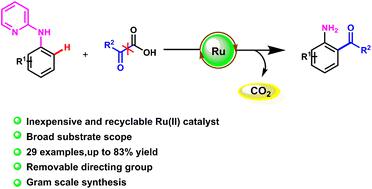
An efficient and convenient synthetic strategy for ruthenium(II)-catalyzed ortho-acylation of N-(2-pyridyl)-anilines using α-oxycarboxylic acids as acyl sources is described. The procedure can smoothly proceed under mild conditions, showing good functional group tolerance. Valuable ortho-acylated aniline products have been obtained with moderate to good yields. Furthermore, the reaction could be easily scaled up to the gram scale.
中文翻译:

使用钌催化剂对苯胺衍生物与α-氧代羧酸进行C-H酰化
描述了一种使用 α-羟基羧酸作为酰基源的钌 ( II ) 催化的N- (2-吡啶基)-苯胺邻位酰化的有效且方便的合成策略。该过程可以在温和的条件下顺利进行,表现出良好的官能团耐受性。以中等至良好的收率获得了有价值的邻酰化苯胺产品。此外,反应可以很容易地放大到克级。
更新日期:2022-08-08
中文翻译:

使用钌催化剂对苯胺衍生物与α-氧代羧酸进行C-H酰化
描述了一种使用 α-羟基羧酸作为酰基源的钌 ( II ) 催化的N- (2-吡啶基)-苯胺邻位酰化的有效且方便的合成策略。该过程可以在温和的条件下顺利进行,表现出良好的官能团耐受性。以中等至良好的收率获得了有价值的邻酰化苯胺产品。此外,反应可以很容易地放大到克级。































 京公网安备 11010802027423号
京公网安备 11010802027423号