Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of water structure in alkaline water electrolysis
iScience ( IF 4.6 ) Pub Date : 2022-08-02 , DOI: 10.1016/j.isci.2022.104835
Anku Guha 1 , Mihir Sahoo 2 , Khorsed Alam 2 , D Krishna Rao 1 , Prasenjit Sen 2 , Tharangattu N Narayanan 1
iScience ( IF 4.6 ) Pub Date : 2022-08-02 , DOI: 10.1016/j.isci.2022.104835
Anku Guha 1 , Mihir Sahoo 2 , Khorsed Alam 2 , D Krishna Rao 1 , Prasenjit Sen 2 , Tharangattu N Narayanan 1
Affiliation
|
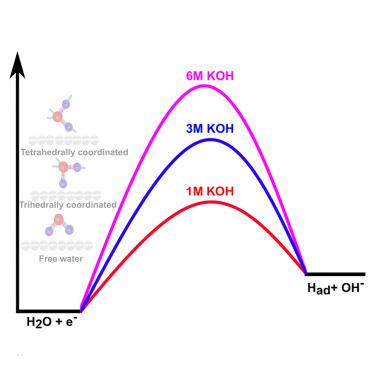
Herein, with the help of experimental and first-principles density functional theory (DFT)-based studies, we have shown that structural changes in the water coordination in electrolytes having high alkalinity can be a possible reason for the reduced catalytic activity of platinum (Pt) in high pH. Studies with Pt electrodes indicate that electrocatalytic HER activity reduces in terms of high overpotential required, high Tafel slope, and high charge transfer resistances in concentrated aqueous alkaline electrolytes (say 6 M KOH) in comparison to that in low alkaline electrolytes (say 0.1 M KOH), irrespective of the counter cations (Na, K, or Rb) present. The changes in the water structure of bulk electrolytes as well as that in electrode-electrolyte interface are studied. The results are compared with DFT-based analysis, and the study can pave new directions in studying the HER process in terms of the water structure near the electrode-electrolyte interface.
中文翻译:

水结构在碱性水电解中的作用
在此,借助实验和基于第一性原理密度泛函理论(DFT)的研究,我们发现高碱度电解质中水配位的结构变化可能是铂(Pt)催化活性降低的可能原因。 )在高pH值下。 Pt 电极的研究表明,与低碱性电解质(例如 0.1 M KOH)相比,在浓碱性电解质水溶液(例如 6 M KOH)中,电催化 HER 活性会因所需的高过电势、高塔菲尔斜率和高电荷转移电阻而降低。 ),与存在的抗衡阳离子(Na、K 或 Rb)无关。研究了本体电解质的水结构以及电极-电解质界面的变化。结果与基于 DFT 的分析进行了比较,该研究可以为研究电极-电解质界面附近的水结构的 HER 过程开辟新的方向。
更新日期:2022-08-02
中文翻译:

水结构在碱性水电解中的作用
在此,借助实验和基于第一性原理密度泛函理论(DFT)的研究,我们发现高碱度电解质中水配位的结构变化可能是铂(Pt)催化活性降低的可能原因。 )在高pH值下。 Pt 电极的研究表明,与低碱性电解质(例如 0.1 M KOH)相比,在浓碱性电解质水溶液(例如 6 M KOH)中,电催化 HER 活性会因所需的高过电势、高塔菲尔斜率和高电荷转移电阻而降低。 ),与存在的抗衡阳离子(Na、K 或 Rb)无关。研究了本体电解质的水结构以及电极-电解质界面的变化。结果与基于 DFT 的分析进行了比较,该研究可以为研究电极-电解质界面附近的水结构的 HER 过程开辟新的方向。

































 京公网安备 11010802027423号
京公网安备 11010802027423号