当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-free highly regioselective hydrated ring-opening and formylation of quinazolinones
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2ob01234k
Xianglin Yu 1 , Zhiliang Tang 1 , Kun He 1 , Weina Li 1 , Jun Lin 1 , Yi Jin 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2ob01234k
Xianglin Yu 1 , Zhiliang Tang 1 , Kun He 1 , Weina Li 1 , Jun Lin 1 , Yi Jin 1
Affiliation
|
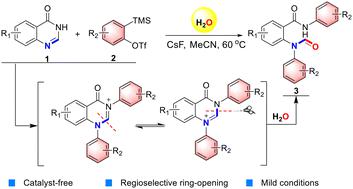
A catalyst-free method for the highly regioselective hydrated ring-opening and formylation of quinazolinones was developed. This reaction realized the direct arylation of two nitrogen atoms on quinazolinones and realized the regioselective ring-opening of quinazolinone and subsequent acylation of methyleneamine through the nucleophilic addition of a water molecule to an imine carbon atom. It showed reasonable functional group compatibility and provided one-pot access to a variety of N-arylformyl derivatives in moderate to excellent yields.
中文翻译:

喹唑啉酮的无催化剂高区域选择性水合开环和甲酰化
开发了一种用于喹唑啉酮类的高区域选择性水合开环和甲酰化的无催化剂方法。该反应实现了喹唑啉酮上两个氮原子的直接芳基化,并通过水分子与亚胺碳原子的亲核加成实现了喹唑啉酮的区域选择性开环和随后的亚甲基胺的酰化。它显示出合理的官能团相容性,并以中等至优异的产率提供了一锅获得各种N-芳基甲酰基衍生物的途径。
更新日期:2022-08-08
中文翻译:

喹唑啉酮的无催化剂高区域选择性水合开环和甲酰化
开发了一种用于喹唑啉酮类的高区域选择性水合开环和甲酰化的无催化剂方法。该反应实现了喹唑啉酮上两个氮原子的直接芳基化,并通过水分子与亚胺碳原子的亲核加成实现了喹唑啉酮的区域选择性开环和随后的亚甲基胺的酰化。它显示出合理的官能团相容性,并以中等至优异的产率提供了一锅获得各种N-芳基甲酰基衍生物的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号