当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and evaluation of carbamate derivatives as fatty acid amide hydrolase and monoacylglycerol lipase inhibitors
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2022-08-03 , DOI: 10.1002/ardp.202200081 Shivani Jaiswal 1 , Garima Gupta 1 , Senthil R Ayyannan 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2022-08-03 , DOI: 10.1002/ardp.202200081 Shivani Jaiswal 1 , Garima Gupta 1 , Senthil R Ayyannan 1
Affiliation
|
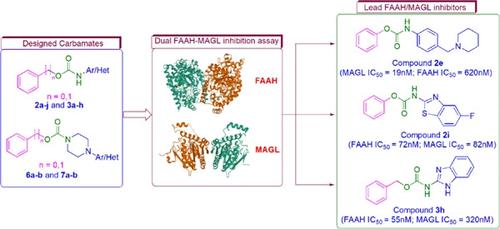
Fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) are the primary catabolic enzymes for endocannabinoids, anandamide (AEA), and 2-arachidonoyl glycerol. Numerous studies have shown that FAAH and MAGL play an important role in modulating various central nervous system activities; hence, the development of small molecule FAAH/MAGL inhibitors is an active area of research. Several small molecules possessing the carbamate scaffold are documented as potential FAAH/MAGL inhibitors. Here, we designed and synthesized a series of open chain and cyclic carbamates and evaluated their dual FAAH–MAGL inhibition properties. Phenyl [4-(piperidin-1-ylmethyl)phenyl]carbamate (2e) emerged as the most potent MAGL inhibitor (IC50 = 19 nM), benzyl (1H-benzo[d]imidazol-2-yl)carbamate (3h) was the most potent FAAH inhibitor (IC50 = 55 nM), and phenyl (6-fluorobenzo[d]thiazol-2-yl)carbamate (2i) egressed as a nonselective dual FAAH–MAGL inhibitor (FAAH: 82 nM, MAGL: 72 nM). The enzyme kinetics experiments revealed that the compounds inhibit FAAH/MAGL in a covalent-reversible manner, with a mixed binding mode of action. Moreover, the lead compounds were found suitable for blood–brain permeation in the parallel artificial membrane permeation assay. Furthermore, docking simulation experiments suggested that the potency of the lead compounds was governed by hydrogen bonds and hydrophobic interactions with the enzyme active sites. In silico drug-likeness and ADMETox prediction studies provided useful information on the compounds' oral absorption, metabolism, and toxicity profiles. In summary, this study afforded potent multifunctional carbamates with appreciable pharmacokinetic profiles meriting further investigation.
中文翻译:

氨基甲酸酯衍生物作为脂肪酸酰胺水解酶和单酰基甘油脂肪酶抑制剂的合成与评价
脂肪酸酰胺水解酶 (FAAH) 和单酰基甘油脂肪酶 (MAGL) 是内源性大麻素、anandamide (AEA) 和 2-花生四烯酸甘油的主要分解代谢酶。大量研究表明,FAAH 和 MAGL 在调节各种中枢神经系统活动方面发挥着重要作用;因此,小分子 FAAH/MAGL 抑制剂的开发是一个活跃的研究领域。几种具有氨基甲酸酯支架的小分子被记录为潜在的 FAAH/MAGL 抑制剂。在这里,我们设计并合成了一系列开链和环状氨基甲酸酯,并评估了它们的双重 FAAH-MAGL 抑制特性。苯基 [4-(哌啶-1-基甲基)苯基]氨基甲酸酯 ( 2e ) 成为最有效的 MAGL 抑制剂 (IC 50 = 19 nM),苄基 (1 H-苯并[d ]imidazol-2-yl)carbamate ( 3h ) 是最有效的 FAAH 抑制剂 (IC 50 = 55 nM),苯基 (6-fluorobenzo[ d ]thiazol-2-yl)carbamate ( 2i) 作为非选择性双重 FAAH-MAGL 抑制剂(FAAH:82 nM,MAGL:72 nM)排出。酶动力学实验表明,这些化合物以共价可逆方式抑制 FAAH/MAGL,具有混合结合模式。此外,在平行人工膜渗透试验中,发现这些先导化合物适用于血脑渗透。此外,对接模拟实验表明,先导化合物的效力受氢键和与酶活性位点的疏水相互作用的控制。计算机模拟药物相似性和 ADMETox 预测研究提供了有关化合物口服吸收、代谢和毒性特征的有用信息。总之,本研究提供了具有明显药代动力学特征的强效多功能氨基甲酸酯,值得进一步研究。
更新日期:2022-08-03
中文翻译:

氨基甲酸酯衍生物作为脂肪酸酰胺水解酶和单酰基甘油脂肪酶抑制剂的合成与评价
脂肪酸酰胺水解酶 (FAAH) 和单酰基甘油脂肪酶 (MAGL) 是内源性大麻素、anandamide (AEA) 和 2-花生四烯酸甘油的主要分解代谢酶。大量研究表明,FAAH 和 MAGL 在调节各种中枢神经系统活动方面发挥着重要作用;因此,小分子 FAAH/MAGL 抑制剂的开发是一个活跃的研究领域。几种具有氨基甲酸酯支架的小分子被记录为潜在的 FAAH/MAGL 抑制剂。在这里,我们设计并合成了一系列开链和环状氨基甲酸酯,并评估了它们的双重 FAAH-MAGL 抑制特性。苯基 [4-(哌啶-1-基甲基)苯基]氨基甲酸酯 ( 2e ) 成为最有效的 MAGL 抑制剂 (IC 50 = 19 nM),苄基 (1 H-苯并[d ]imidazol-2-yl)carbamate ( 3h ) 是最有效的 FAAH 抑制剂 (IC 50 = 55 nM),苯基 (6-fluorobenzo[ d ]thiazol-2-yl)carbamate ( 2i) 作为非选择性双重 FAAH-MAGL 抑制剂(FAAH:82 nM,MAGL:72 nM)排出。酶动力学实验表明,这些化合物以共价可逆方式抑制 FAAH/MAGL,具有混合结合模式。此外,在平行人工膜渗透试验中,发现这些先导化合物适用于血脑渗透。此外,对接模拟实验表明,先导化合物的效力受氢键和与酶活性位点的疏水相互作用的控制。计算机模拟药物相似性和 ADMETox 预测研究提供了有关化合物口服吸收、代谢和毒性特征的有用信息。总之,本研究提供了具有明显药代动力学特征的强效多功能氨基甲酸酯,值得进一步研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号