Molecular Catalysis ( IF 3.9 ) Pub Date : 2022-08-07 , DOI: 10.1016/j.mcat.2022.112560 Josefredo R. Pliego
|
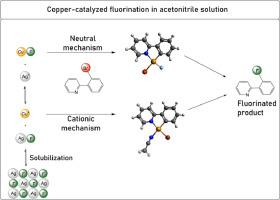
Nucleophilic fluorination via copper catalysis has been a challenging transformation and only special substrates can react. One example is the 2-(2-bromophenyl)pyridine, which was fluorinated with AgF using CuPF6 catalyst in acetonitrile solvent. In this work, a detailed analysis of this catalyzed reaction was done using theoretical methods. The free Cu+ ion and the CuF ion pair in acetonitrile solvent were modeled using a quasi-chemical cluster-continuum approach and electronic energies calculated at DLPNO-CCSD(T) level with a quadruple-zeta basis set. The catalytic cycle involves the Cu(I)/Cu(III) mechanism via cationic (Cu+ ion) or neutral (CuF ion pair) pathways. The solubility of AgF and CuF was included in the analysis, as well as the transmetalation step. The calculated free energy profile was used to perform a microkinetic analysis of the reaction system. The results point out that the reaction proceeds via the cationic mechanism, with the formation of the catalyst-substrate complex followed by the oxidative addition step with ΔG‡ = 30.8 kcal mol−1. The formed intermediate is high in free energy and the reductive elimination step is the rate-determining one, with an overall ΔG‡ = 32.6 kcal mol−1. Analysis of the free energy profile and the microkinetic modeling point out almost zero-order kinetics in substrate concentration when the catalyst is used in substoichiometric quantity. Approximated experimental ΔG‡ is estimated as being 30.7 kcal mol−1, in very good agreement with the theoretical value. The neutral mechanism with the soluble CuF ion pair has ΔG‡ = 33.4 kcal mol−1, which is competitive with the cationic mechanism. However, due to the low solubility and concentration of this ion pair, the reaction does not take place by this pathway.
中文翻译:

乙腈溶剂中铜催化 2-(2-溴苯基)吡啶通过 Cu(I)/Cu(III) 芳族氟化:簇连续自由能分布和微动力学分析
通过铜催化进行的亲核氟化一直是一项具有挑战性的转化,只有特殊的底物才能发生反应。一个例子是 2-(2-溴苯基)吡啶,它在乙腈溶剂中使用 CuPF 6催化剂用 AgF 氟化。在这项工作中,使用理论方法对该催化反应进行了详细分析。乙腈溶剂中的游离 Cu +离子和 CuF 离子对使用准化学簇连续谱方法建模,并在 DLPNO-CCSD(T) 水平上计算电子能量,并使用四 zeta 基组。催化循环涉及通过阳离子(Cu +离子)或中性(CuF 离子对)途径。分析中包括 AgF 和 CuF 的溶解度以及金属转移步骤。计算的自由能分布用于对反应系统进行微动力学分析。结果表明,反应通过阳离子机理进行,形成催化剂-底物复合物,然后进行氧化加成步骤,ΔG ‡ = 30.8 kcal mol -1。形成的中间体具有高自由能,还原消除步骤是决定速率的步骤,总 ΔG ‡ = 32.6 kcal mol -1. 对自由能分布和微动力学模型的分析指出,当催化剂以亚化学计量使用时,底物浓度几乎为零级动力学。近似的实验ΔG ‡估计为30.7 kcal mol -1,与理论值非常吻合。具有可溶性 CuF 离子对的中性机制具有 ΔG ‡ = 33.4 kcal mol -1,这与阳离子机制具有竞争性。然而,由于该离子对的溶解度和浓度低,反应不会通过该途径发生。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号