Phytochemistry ( IF 3.2 ) Pub Date : 2022-08-05 , DOI: 10.1016/j.phytochem.2022.113356
Bryan J Leong 1 , Jacob S Folz 2 , Ulschan Bathe 1 , David G Clark 3 , Oliver Fiehn 2 , Andrew D Hanson 1
|
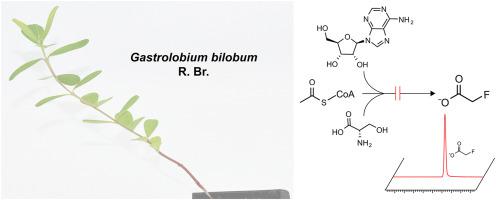
Like angiosperms from several other families, the leguminous shrub Gastrolobium bilobum R.Br. produces and accumulates fluoroacetate, indicating that it performs the difficult chemistry needed to make a C–F bond. Bioinformatic analyses indicate that plants lack homologs of the only enzymes known to make a C–F bond, i.e., the Actinomycete flurorinases that form 5′-fluoro-5′-deoxyadenosine from S-adenosylmethionine and fluoride ion. To probe the origin of fluoroacetate in G. bilobum we first showed that fluoroacetate accumulates to millimolar levels in young leaves but not older leaves, stems or roots, that leaf fluoroacetate levels vary >20-fold between individual plants and are not markedly raised by sodium fluoride treatment. Young leaves were fed adenosine-13C-ribose, 13C-serine, or 13C-acetate to test plausible biosynthetic routes to fluoroacetate from S-adenosylmethionine, a C3-pyridoxal phosphate complex, or acetyl-CoA, respectively. Incorporation of 13C into expected metabolites confirmed that all three precursors were taken up and metabolized. Consistent with the bioinformatic evidence against an Actinomycete-type pathway, no adenosine-13C-ribose was converted to 13C-fluoroacetate; nor was the characteristic 4-fluorothreonine product of the Actinomycete pathway detected. Similarly, no 13C from acetate or serine was incorporated into fluoroacetate. While not fully excluding the hypothetical pathways that were tested, these negative labeling data imply that G. bilobum creates the C–F bond by an unprecedented biochemical reaction. Enzyme(s) that mediate such a reaction could be of great value in pharmaceutical and agrochemical manufacturing.
中文翻译:

氟乙酸盐的分布、对氟化的反应,以及在幼年 Gastrolobium bilobum 植物中的合成
与其他几个科的被子植物一样,豆科灌木Gastrolobium bilobum R.Br. 产生并积累氟乙酸盐,表明它执行了形成 C-F 键所需的困难化学反应。生物信息学分析表明,植物缺乏唯一已知的形成 C-F 键的酶的同源物,即从S-腺苷甲硫氨酸和氟离子形成 5'-氟-5'-脱氧腺苷的放线菌氟脲酶。为了探究G. bilobum中氟乙酸盐的来源,我们首先表明氟乙酸盐在幼叶而不是老叶、茎或根中积累到毫摩尔水平,叶氟乙酸盐水平在单个植物之间变化 > 20 倍,并且钠不会显着提高氟化物处理。幼叶被喂食腺苷-13 C-核糖、13 C-丝氨酸或13 C-乙酸盐分别测试从S-腺苷甲硫氨酸、C 3 -磷酸吡哆醛复合物或乙酰辅酶 A 到氟乙酸盐的合理生物合成途径。将13 C 结合到预期的代谢物中证实了所有三种前体都被吸收和代谢。与针对放线菌类途径的生物信息学证据一致,没有腺苷- 13 C-核糖转化为13 C-氟乙酸盐;也没有检测到放线菌途径的特征性 4-氟苏氨酸产物。同样,没有13来自乙酸盐或丝氨酸的 C 并入氟乙酸盐中。虽然没有完全排除所测试的假设途径,但这些负面标记数据暗示G. bilobum通过前所未有的生化反应产生 C-F 键。介导这种反应的酶在制药和农用化学品制造中可能具有重要价值。

































 京公网安备 11010802027423号
京公网安备 11010802027423号