Cell Reports ( IF 7.5 ) Pub Date : 2022-08-03 , DOI: 10.1016/j.celrep.2022.111168 David Millrine 1 , Thomas Cummings 1 , Stephen P Matthews 1 , Joshua J Peter 1 , Helge M Magnussen 1 , Sven M Lange 1 , Thomas Macartney 1 , Frederic Lamoliatte 1 , Axel Knebel 1 , Yogesh Kulathu 1
|
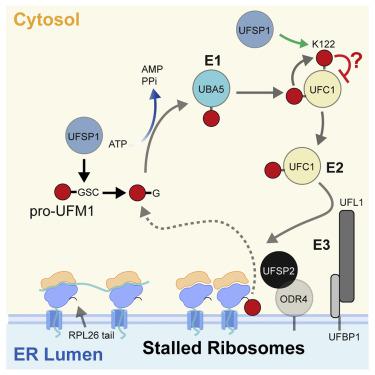
An essential first step in the post-translational modification of proteins with UFM1, UFMylation, is the proteolytic cleavage of pro-UFM1 to expose a C-terminal glycine. Of the two UFM1-specific proteases (UFSPs) identified in humans, only UFSP2 is reported to be active, since the annotated sequence of UFSP1 lacks critical catalytic residues. Nonetheless, efficient UFM1 maturation occurs in cells lacking UFSP2, suggesting the presence of another active protease. We herein identify UFSP1 translated from a non-canonical start site to be this protease. Cells lacking both UFSPs show complete loss of UFMylation resulting from an absence of mature UFM1. While UFSP2, but not UFSP1, removes UFM1 from the ribosomal subunit RPL26, UFSP1 acts earlier in the pathway to mature UFM1 and cleave a potential autoinhibitory modification on UFC1, thereby controlling activation of UFMylation. In summary, our studies reveal important distinctions in substrate specificity and localization-dependent functions for the two proteases in regulating UFMylation.
中文翻译:

人类 UFSP1 是一种活性蛋白酶,可调节 UFM1 成熟和 UFMylation
使用 UFM1 对蛋白质进行翻译后修饰(UFMylation)的重要第一步是对 UFM1 前体进行蛋白水解切割,以暴露 C 末端甘氨酸。在人体中鉴定的两种 UFM1 特异性蛋白酶 (UFSP) 中,据报道只有 UFSP2 具有活性,因为 UFSP1 的注释序列缺乏关键的催化残基。尽管如此,缺乏 UFSP2 的细胞中却发生了有效的 UFM1 成熟,表明存在另一种活性蛋白酶。我们在此鉴定从非规范起始位点翻译的 UFSP1 就是这种蛋白酶。缺乏两个 UFSP 的细胞表现出由于成熟 UFM1 缺失而导致 UFMylation 完全丧失。虽然 UFSP2(而非 UFSP1)从核糖体亚基 RPL26 中去除 UFM1,但 UFSP1 在成熟 UFM1 途径中较早发挥作用,并裂解 UFC1 上潜在的自抑制修饰,从而控制 UFMylation 的激活。总之,我们的研究揭示了两种蛋白酶在调节 UFMylation 方面底物特异性和定位依赖性功能的重要区别。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号