当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a New Synthetic Route of the Key Intermediate of Irbesartan
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.oprd.2c00113 Zhaohang Chen 1 , Yuanxia Guo 1 , Huilong Niu 1 , Jinxin Wang 1 , Jianming Li 1 , Chao Li 1 , Renzhong Qiao 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.oprd.2c00113 Zhaohang Chen 1 , Yuanxia Guo 1 , Huilong Niu 1 , Jinxin Wang 1 , Jianming Li 1 , Chao Li 1 , Renzhong Qiao 1
Affiliation
|
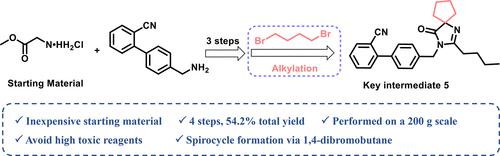
Herein, we describe a new synthetic route to prepare 4′-((2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl)-[1,1′-biphenyl]-2-carbonitrile (5), an intermediate of irbesartan. Compared with leucine or its derivatives as the starting material to synthesize irbesartan in many previous reports, the intermediate 5 could be obtained in four steps from low-cost and commercially available glycine methyl ester as the starting material, which also avoided the use of highly toxic cyanide. The spirocycle structure in the intermediate 5 was constructed with dihaloalkanes via a simple alkylation reaction. The related impurities and process parameters were studied in detail. Finally, the scale-up of this route was successfully performed on a 200 g scale, affording 332 g of the intermediate 5 with 98.4% purity and 54.2% overall yield in four steps. Meanwhile, process mass intensity and yield stability were demonstrated. The current synthetic route provides an alternative strategy for the production of irbesartan.
中文翻译:

厄贝沙坦关键中间体合成新路线的开发
在此,我们描述了一种制备 4'-((2-丁基-4-氧代-1,3-二氮杂螺[4.4]non-1-en-3-yl)methyl)-[1,1'-联苯]-2-甲腈( 5 ),厄贝沙坦的中间体。与以往多篇报道以亮氨酸或其衍生物为起始原料合成厄贝沙坦相比,中间体5可以以低成本、市售的甘氨酸甲酯为起始原料,四步制得,也避免了使用剧毒。氰化物。中间体5中的螺环结构由二卤代烷通过一个简单的烷基化反应。对相关杂质及工艺参数进行了详细研究。最后,该路线的放大在 200 g 规模上成功进行,在四个步骤中提供了 332 g 纯度为 98.4% 和总产率为 54.2%的中间体5 。同时,工艺质量强度和产量稳定性得到了证明。目前的合成路线为厄贝沙坦的生产提供了一种替代策略。
更新日期:2022-08-04
中文翻译:

厄贝沙坦关键中间体合成新路线的开发
在此,我们描述了一种制备 4'-((2-丁基-4-氧代-1,3-二氮杂螺[4.4]non-1-en-3-yl)methyl)-[1,1'-联苯]-2-甲腈( 5 ),厄贝沙坦的中间体。与以往多篇报道以亮氨酸或其衍生物为起始原料合成厄贝沙坦相比,中间体5可以以低成本、市售的甘氨酸甲酯为起始原料,四步制得,也避免了使用剧毒。氰化物。中间体5中的螺环结构由二卤代烷通过一个简单的烷基化反应。对相关杂质及工艺参数进行了详细研究。最后,该路线的放大在 200 g 规模上成功进行,在四个步骤中提供了 332 g 纯度为 98.4% 和总产率为 54.2%的中间体5 。同时,工艺质量强度和产量稳定性得到了证明。目前的合成路线为厄贝沙坦的生产提供了一种替代策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号