Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2022-08-01 , DOI: 10.1016/j.bmc.2022.116952 Sarah Macedo Vaz 1 , Matheus de Freitas Silva 1 , Graziella Dos Reis Rosa Franco 1 , Marcos Jorge R Guimarães 2 , Fernanda Motta R da Silva 2 , Newton Gonçalves Castro 2 , Isabella Alvim Guedes 3 , Laurent E Dardenne 3 , Marina Amaral Alves 4 , Rafael Garrett da Costa 4 , Gabriela Beserra Pinheiro 5 , Letícia Germino Veras 5 , Márcia Renata Mortari 5 , Letizia Pruccoli 6 , Andrea Tarozzi 6 , Cláudio Viegas 1
|
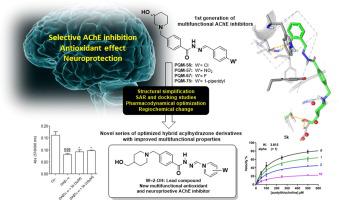
The search for new drug candidates against Alzheimer’s disease (AD) remains a complex challenge for medicinal chemists due to its multifactorial pathogenesis and incompletely understood physiopathology. In this context, we have explored the molecular hybridization of pharmacophore structural fragments from known bioactive molecules, aiming to obtain a novel molecular architecture in new chemical entities capable of concomitantly interacting with multiple targets in a so-called multi-target directed ligands (MTDLs) approach. This work describes the synthesis of 4-hydroxymethyl)piperidine-N-benzyl-acyl-hydrazone derivatives 5a-l, designed as novel MTDLs, showing improved multifunctional properties compared to the previously reported parent series of N-benzyl-(3-hydroxy)piperidine-acyl-hydrazone derivatives 4. The new improved derivatives were studied in silico, regarding their mode of interaction with AChE enzyme, and in vitro, for evaluation of their effects on the selective inhibition of cholinesterases, cellular antioxidant, and neuroprotective activities as their cytotoxicity in human neuroblastoma (SH-SY5Y) cells. Overall, compound PQM-181 (5 k) showed the best balanced selective and non-competitive inhibition of AChE (IC50 = 5.9 μM, SI > 5.1), with an additional antioxidant activity (IC50 = 7.45 µM) against neuronal t-BOOH-induced oxidative stress and neuroprotective ability against neurotoxicity elicited by both t-BOOH and OAβ1-42, and a moderate ability to interfere in Aβ1-42 aggregates, with low cytotoxicity and good predictive druggability properties, suggesting a multifunctional pharmacological profile suitable for further drug development against AD.
中文翻译:

4-羟基-甲基哌啶基-N-苄基-酰基芳基腙杂化物的合成和生物学评价设计为阿尔茨海默病的新型多功能候选药物
由于其多因素发病机制和不完全了解的病理生理学,寻找针对阿尔茨海默病 (AD) 的新候选药物仍然是药物化学家面临的一项复杂挑战。在这种情况下,我们探索了来自已知生物活性分子的药效团结构片段的分子杂交,旨在在新的化学实体中获得一种新的分子结构,能够与所谓的多靶点定向配体 (MTDLs) 中的多个靶点同时相互作用方法。这项工作描述了 4-羟甲基)哌啶-N-苄基-酰基-腙衍生物5a-l的合成,设计为新型 MTDL,与先前报道的N母体系列相比,显示出改进的多功能特性-苄基-(3-羟基)哌啶-酰基-腙衍生物4.在计算机上研究了新的改进衍生物与 AChE 酶的相互作用模式,并在体外研究了它们对胆碱酯酶的选择性抑制、细胞抗氧化和神经保护活性的影响,因为它们在人神经母细胞瘤中的细胞毒性 (SH-SY5Y ) 细胞。总体而言,化合物 PQM-181 ( 5 k ) 对 AChE 具有最佳平衡的选择性和非竞争性抑制作用 (IC 50 = 5.9 μM, SI > 5.1),并具有额外的抗氧化活性 (IC 50 = 7.45 μM) 对神经元t - BOOH诱导的氧化应激和对神经毒性的神经保护能力-BOOH 和 OAβ 1-42 ,以及对 Aβ 1-42聚集体的适度干扰能力,具有低细胞毒性和良好的预测成药性,表明适合进一步开发针对 AD 的药物的多功能药理学特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号