Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2022-08-03 , DOI: 10.1016/j.freeradbiomed.2022.07.015 Jun Chen 1 , Chun-Yan Fu 2 , Gerong Shen 2 , Jingyu Wang 3 , Lintao Xu 3 , Heyangzi Li 2 , Xi Cao 2 , Ming-Zhi Zheng 4 , Yue-Liang Shen 2 , Jinjie Zhong 5 , Ying-Ying Chen 5 , Lin-Lin Wang 1
|
Introduction
Mitochondrial transfer is a new cell-to-cell communication manner. Whether the mitochondrial transfer is also involved in the macrophage infiltration-induced cardiac injury is unclear.
Objectives
This study aimed to determine whether macrophage mitochondria can be transferred to cardiomyocytes, and to investigate its possible role and mechanism.
Methods
Mitochondrial transfer between macrophages and cardiomyocytes was detected using immunofluorescence staining and flow cytometry. Cellular metabolites were analyzed using LC-MS technique. Differentially expressed mRNAs were identified using RNA-seq technique.
Results
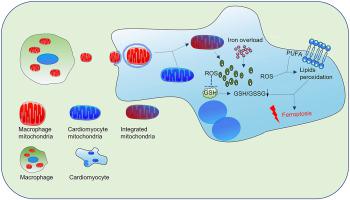
(1) After cardiomyocytes were cultured with macrophage-conditioned medium (COND + group), macrophage-derived mitochondria have been found in cardiomyocytes, which could be blocked by dynasore (an inhibitor of clathrin-mediated endocytosis). (2) Compared with control (CM) group, there were 545 altered metabolites found in COND + group, most of which were lipids and lipid-like molecules. The altered metabolites were mainly enriched in the β-oxidation of fatty acids and glutathione metabolism. And there were 4824 differentially expressed mRNAs, which were highly enriched in processes like lipid metabolism-associated pathway. (3) Both RNA-seq and qRT-PCR results found that ferroptosis-related mRNAs such as Ptgs2 and Acsl4 increased, and Gpx4 mRNA decreased in COND + group (P < 0.05 vs CM group). (4) The levels of cellular free Fe2+ and mitochondrial lipid peroxidation were increased; while GSH/GSSG ratio, mitochondrial aspect ratio, mitochondrial membrane potential, and ATP production were decreased in cardiomyocytes of COND + group (P < 0.05 vs CM group). All the above phenomena could be blocked by a ferroptosis inhibitor ferrostatin-1 (P < 0.05).
Conclusion
Macrophages could transfer mitochondria to cardiomyocytes. Macrophage-derived mitochondria were internalized into cardiomyocytes through clathrin- and/or lipid raft-mediated endocytosis. Uptake of exogenous macrophage mitochondria induced cardiomyocyte injury via triggering ferroptosis.
中文翻译:

巨噬细胞通过线粒体转移诱导心肌细胞铁死亡
介绍
线粒体转移是一种新的细胞间通讯方式。线粒体转移是否也参与巨噬细胞浸润诱导的心脏损伤尚不清楚。
目标
本研究旨在确定巨噬细胞线粒体是否可以转移到心肌细胞,并探讨其可能的作用和机制。
方法
使用免疫荧光染色和流式细胞术检测巨噬细胞和心肌细胞之间的线粒体转移。使用 LC-MS 技术分析细胞代谢物。使用 RNA-seq 技术鉴定差异表达的 mRNA。
结果
(1) 用巨噬细胞条件培养基(COND + 组)培养心肌细胞后,在心肌细胞中发现了巨噬细胞来源的线粒体,它可以被 dynasore(一种网格蛋白介导的内吞作用的抑制剂)阻断。(2)与对照组(CM)组相比,COND+组有545种代谢物发生改变,其中大部分是脂质和类脂分子。改变的代谢物主要富集于脂肪酸的β-氧化和谷胱甘肽代谢。并且有4824个差异表达的mRNA,它们在脂质代谢相关途径等过程中高度富集。(3)RNA-seq和qRT-PCR结果均发现Ptgs2、Acsl4等铁死亡相关mRNAs增加,Gpx4COND + 组 mRNA 降低(与CM 组相比P < 0.05 )。(4)细胞游离Fe 2+和线粒体脂质过氧化水平升高;而COND+组心肌细胞的GSH/GSSG比值、线粒体纵横比、线粒体膜电位和ATP生成降低(P < 0.05 vs CM组)。所有上述现象均可被铁死亡抑制剂 ferrostatin-1 阻断(P < 0.05)。
结论
巨噬细胞可以将线粒体转移到心肌细胞。巨噬细胞衍生的线粒体通过网格蛋白和/或脂筏介导的内吞作用被内化到心肌细胞中。外源性巨噬细胞线粒体的摄取通过触发铁死亡诱导心肌细胞损伤。































 京公网安备 11010802027423号
京公网安备 11010802027423号