Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanocarrier Co-formulation for Delivery of a TLR7 Agonist plus an Immunogenic Cell Death Stimulus Triggers Effective Pancreatic Cancer Chemo-immunotherapy
ACS Nano ( IF 15.8 ) Pub Date : 2022-08-03 , DOI: 10.1021/acsnano.2c06300 Lijia Luo 1, 2 , Xiang Wang 1, 2 , Yu-Pei Liao 1 , Chong Hyun Chang 2 , Andre E Nel 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2022-08-03 , DOI: 10.1021/acsnano.2c06300 Lijia Luo 1, 2 , Xiang Wang 1, 2 , Yu-Pei Liao 1 , Chong Hyun Chang 2 , Andre E Nel 1, 2
Affiliation
|
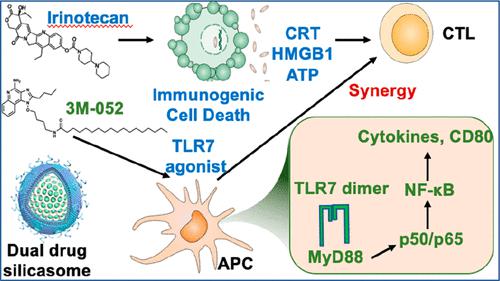
Although toll-like receptor (TLR) agonists hold great promise as immune modulators for reprogramming the suppressive immune landscape in pancreatic ductal adenocarcinoma (PDAC), their use is limited by poor pharmacokinetics (PK) and off-target systemic inflammatory effects. To overcome these challenges as well as to attain drug synergy, we developed a lipid bilayer (LB)-coated mesoporous silica nanoparticle (silicasome) platform for co-delivery of the TLR7/8 agonist 3M-052 with the immunogenic chemotherapeutic agent irinotecan. This was accomplished by incorporating the C18 lipid tail of 3M-052 in the coated LB, also useful for irinotecan remote loading in the porous interior. Not only did the co-formulated carrier improve PK, but it strengthened the irinotecan-induced immunogenic cell death response by 3M-052-mediated dendritic cell activation at the tumor site as well as participating lymph nodes. The accompanying increase in CD8+ T-cell infiltration along with a reduced number of regulatory T-cells was associated with tumor shrinkage and metastasis disappearance in subcutaneous and orthotopic KRAS-mediated pancreatic carcinoma tumor models. Moreover, this therapeutic outcome was accomplished without drug or nanocarrier toxicity. All considered, dual-delivery strategies that combine chemo-immunotherapy with co-formulated TLR agonists or other lipid-soluble immune modulators predict successful intervention in heterogeneous PDAC immune landscapes.
中文翻译:

用于递送 TLR7 激动剂和免疫原性细胞死亡刺激物的纳米载体复合制剂可触发有效的胰腺癌化学免疫疗法
尽管 Toll 样受体 (TLR) 激动剂作为免疫调节剂在重新编程胰腺导管腺癌 (PDAC) 的抑制性免疫景观方面具有广阔的前景,但其使用受到较差的药代动力学 (PK) 和脱靶全身炎症效应的限制。为了克服这些挑战并实现药物协同作用,我们开发了一种脂质双层 (LB) 包被的介孔二氧化硅纳米颗粒(二氧化硅体)平台,用于共同递送 TLR7/8 激动剂 3M-052 与免疫原性化疗药物伊立替康。这是通过将 3M-052 的 C18 脂质尾部合并到涂层 LB 中来实现的,这也可用于多孔内部的伊立替康远程加载。联合配制的载体不仅改善了 PK,而且通过 3M-052 介导的肿瘤部位和参与淋巴结的树突状细胞激活,增强了伊立替康诱导的免疫原性细胞死亡反应。在皮下和原位 KRAS 介导的胰腺癌肿瘤模型中,CD8 + T 细胞浸润的增加以及调节性 T 细胞数量的减少与肿瘤缩小和转移消失相关。此外,这种治疗结果是在没有药物或纳米载体毒性的情况下实现的。综合考虑,将化学免疫疗法与共同配制的 TLR 激动剂或其他脂溶性免疫调节剂相结合的双重递送策略预示着对异质 PDAC 免疫环境的成功干预。
更新日期:2022-08-03
中文翻译:

用于递送 TLR7 激动剂和免疫原性细胞死亡刺激物的纳米载体复合制剂可触发有效的胰腺癌化学免疫疗法
尽管 Toll 样受体 (TLR) 激动剂作为免疫调节剂在重新编程胰腺导管腺癌 (PDAC) 的抑制性免疫景观方面具有广阔的前景,但其使用受到较差的药代动力学 (PK) 和脱靶全身炎症效应的限制。为了克服这些挑战并实现药物协同作用,我们开发了一种脂质双层 (LB) 包被的介孔二氧化硅纳米颗粒(二氧化硅体)平台,用于共同递送 TLR7/8 激动剂 3M-052 与免疫原性化疗药物伊立替康。这是通过将 3M-052 的 C18 脂质尾部合并到涂层 LB 中来实现的,这也可用于多孔内部的伊立替康远程加载。联合配制的载体不仅改善了 PK,而且通过 3M-052 介导的肿瘤部位和参与淋巴结的树突状细胞激活,增强了伊立替康诱导的免疫原性细胞死亡反应。在皮下和原位 KRAS 介导的胰腺癌肿瘤模型中,CD8 + T 细胞浸润的增加以及调节性 T 细胞数量的减少与肿瘤缩小和转移消失相关。此外,这种治疗结果是在没有药物或纳米载体毒性的情况下实现的。综合考虑,将化学免疫疗法与共同配制的 TLR 激动剂或其他脂溶性免疫调节剂相结合的双重递送策略预示着对异质 PDAC 免疫环境的成功干预。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号